Oxidative stress, antioxidants and stress tolerance
抗氧化剂抗氧化能力测定法

抗氧化剂抗氧化能力测定法English Answer:Antioxidant Capacity Assay Methods.Antioxidant capacity assays are widely used to evaluate the ability of a substance to inhibit oxidation. Various methods have been developed to measure antioxidant capacity, each with its own advantages and limitations. Here are some commonly used methods:(1) DPPH Assay (2,2-diphenyl-1-picrylhydrazyl)。
The DPPH assay is a simple and widely used method to measure the radical scavenging capacity of antioxidants. In this assay, DPPH, a stable free radical, is reduced to its non-radical form by antioxidants, leading to a color change from purple to yellow. The reduction rate and extent are proportional to the antioxidant capacity of the sample.(2) FRAP Assay (Ferric Reducing Antioxidant Power)。
The FRAP assay measures the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+). Antioxidants reduce Fe3+ to Fe2+ in the presence of a chromogenic substrate, resulting in a color change that can be quantified spectrophotometrically. The FRAP assay is commonly used to assess the reducing power of antioxidants.(3) ABTS Assay (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid))。
2012-Marine Carotenoids and Oxidative Stress=FX=抗氧化
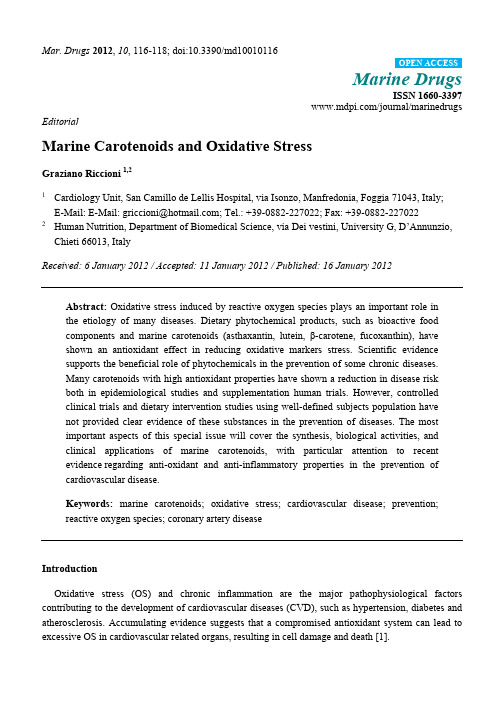
Mar. Drugs 2012, 10, 116-118; doi:10.3390/md10010116OPEN ACCESSMarine DrugsISSN 1660-3397/journal/marinedrugs EditorialMarine Carotenoids and Oxidative StressGraziano Riccioni 1,21Cardiology Unit, San Camillo de Lellis Hospital, via Isonzo, Manfredonia, Foggia 71043, Italy;E-Mail: E-Mail: griccioni@; Tel.: +39-0882-227022; Fax: +39-0882-2270222 Human Nutrition, Department of Biomedical Science, via Dei vestini, University G, D’Annunzio,Chieti 66013, ItalyReceived: 6 January 2012 / Accepted: 11 January 2012 / Published: 16 January 2012Abstract: Oxidative stress induced by reactive oxygen species plays an important role inthe etiology of many diseases. Dietary phytochemical products, such as bioactive foodcomponents and marine carotenoids (asthaxantin, lutein, β-carotene, fucoxanthin), haveshown an antioxidant effect in reducing oxidative markers stress. Scientific evidencesupports the beneficial role of phytochemicals in the prevention of some chronic diseases.Many carotenoids with high antioxidant properties have shown a reduction in disease riskboth in epidemiological studies and supplementation human trials. However, controlledclinical trials and dietary intervention studies using well-defined subjects population havenot provided clear evidence of these substances in the prevention of diseases. The mostimportant aspects of this special issue will cover the synthesis, biological activities, andclinical applications of marine carotenoids, with particular attention to recentevidence regarding anti-oxidant and anti-inflammatory properties in the prevention ofcardiovascular disease.Keywords: marine carotenoids; oxidative stress; cardiovascular disease; prevention;reactive oxygen species; coronary artery diseaseIntroductionOxidative stress (OS) and chronic inflammation are the major pathophysiological factors contributing to the development of cardiovascular diseases (CVD), such as hypertension, diabetes and atherosclerosis. Accumulating evidence suggests that a compromised antioxidant system can lead to excessive OS in cardiovascular related organs, resulting in cell damage and death [1].Emerging evidence suggests that interventions, including nutrition, pharmacology, and physical exercise, may activate expression of cellular anti-oxidant systems and play a role in preventing inflammatory processes in CVD [2]. For these reasons new effective interventions, based on nutrition, aimed at targeting OS and chronic inflammation, may induce an important protection from CVD [3,4].Advances in pathophysiological research suggest that CVD represent a continuum of pathophysiological processes that advance from local redox imbalance to endothelial dysfunction, endothelial inflammation, and excessive vascular remodeling. Consequent cell damage contributes to atherosclerosis, coronary artery disease (CAD), stroke and myocardial infarction [5,6]. In particular CVD are associated with increased production of reactive oxygen species (ROS) and compromised endogenous anti-oxidant defense systems (superoxide dismutases (SODs), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO-1), catalase, and thioredoxin). OS is tightly regulated by a balance between production and removal of ROS. A compromised anti-oxidant defense system can lead to excessive oxidative stress and ultimately result in cell damage [7].The nutritional prevention of atherosclerosis with the use of natural antioxidants represents an important new frontier in the prevention and treatment of CVD. There is evidence that the production of oxidized LDL (LDL OX) can be counteracted by the activity of dietary free radical electron (“antioxidant”) acceptor molecules (such as β-carotene and ascorbic acid), which sequester free radical electrons and prevent the oxidation of LDL particles [8]. Many foods typical of the Mediterranean Diet (such as olive oil, red wine, fruits, and vegetables) contain a mixture of phytonutrients that are both water-soluble and lipid-soluble and can enhance antioxidant capacity throughout the organism, and variations of that dietary regimen are even more effective. For example, several naturally-occurring phytochemicals with antioxidant actions have been associated with the prevention of atherosclerosis, including the carotenoids, lycopene, lutein and astaxanthin, and glabridin, the major isoflavan obtained from licorice roots, even if this evidence is derived from studies presenting an important limitation due to the small number of subjects [9].Although a large body of research has focused on individual or small numbers of antioxidants, increasing circulating antioxidant capacity through increased consumption of antioxidant-rich fruits and vegetables is protective against cardiovascular disease. The available scientific evidence indicates that the link between oxidative stress, a proinflammatory systemic environment and cardiovascular disease is strong [10]. This conclusion can provide increased motivation for dietary improvements that shift the risk equation away from premature death and toward increased longevity and enhanced quality of life.References1 Il’yasova, D.; Ivanova, A.; Morrow, J.D.; Cesari, M.; Pahor, M. Correlation between two markersof inflammation, serum C-reactive protein and interleukin 6, and indices of oxidative stress in patients with high risk of cardiovascular disease. Atherosclerosis2009, 204, 309–314.2 Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscl. Rep.2009; 11, 434–439.3 Riccioni, G.; D'Orazio, N.; Franceschelli, S.; Speranza, L. Marine carotenoids and cardiovascularrisk markers. Mar. Drugs2011, 9, 1166–1175.4 Houston, M.C. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidantsand minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther.Adv. Cardiovasc. Dis.2010, 4, 165–183.5 Chuang, G.C.; Yang, Z.; Westbrook, D.G.; Pompilius, M.; Ballinger, C.A.; White, C.R.;Krzywanski, D.M.; Postlethwait, E.M.; Ballinger, S.W. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell. Mol.Physiol.2009, 297, 209–216.6 Gori, T.; Nzel, T.M. Oxidative stress and endothelial dysfunction: therapeutic implications.Ann. Med. 2011, 43, 259–272.7 Lee, S.; Park, Y.; Zuidema, M.Y.; Hannink, M.; Zhang, C. Effects of interventions on oxidativestress and inflammation of cardiovascular diseases. World J. Cardiol. 2011, 3, 18–24.8 Seo, H.; Oh, H.; Park, H.; Park, M.; Jang, Y.; Lee, M. Contribution of dietary intakes ofantioxidants to homocysteine-induced low density lipoprotein (LDL) oxidation in atherosclerotic patients. Yonsei. Med. J.2010, 51, 526–333.9 Bhatt, D.L. Anti-inflammatory agents and antioxidants as a possible “third great wave” incardiovascular secondary prevention. Am. J. Cardiol.2008, 101, 4–13.10 Fassett, R.G.; Coombes, J.S. Astaxanthin: a potential therapeutic agent in cardiovascular disease.Mar. Drugs2011, 9, 447–465.© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (/licenses/by/3.0/).。
介绍龙眼英文作文带翻译

介绍龙眼英文作文带翻译 英文,Longan, also known as "dragon eye," is a tropical fruit native to Southern Asia. It belongs to the soapberry family, Sapindaceae, and is botanically called Dimocarpus longan. The name "dragon eye" comes from the appearance of the fruit when it's peeled, resembling an eyeball with a black seed inside, surrounded by translucent, juicy flesh.
Longan is a small, round fruit, about the size of a ping-pong ball. Its thin, brownish skin is easily peeled to reveal the juicy, sweet flesh inside. The flavor is often described as floral, with hints of honey and musk. It's commonly enjoyed fresh, but can also be dried, canned, or made into various desserts and beverages.
In Chinese culture, longan holds symbolic significance and is often associated with good fortune and prosperity. It's a popular gift during holidays and celebrations, symbolizing family unity and happiness. For example, during the Chinese New Year, it's common to serve longan alongside other fruits as part of the festivities. Aside from its cultural significance, longan also boasts several health benefits. It's a good source of vitamins and minerals, including vitamin C, vitamin A, potassium, and magnesium. Additionally, it contains antioxidants that help protect the body from oxidative stress and inflammation. Some traditional Chinese medicine practitioners believe that longan can improve blood circulation, strengthen the immune system, and even promote relaxation and better sleep.
氧化态势 英文

氧化态势英文Oxidation SituationThe world we live in is a delicate balance of various chemical reactions, and one of the most crucial among them is the process of oxidation. Oxidation, a fundamental chemical reaction, plays a pivotal role in shaping the very fabric of our existence, from the air we breathe to the energy that powers our lives. In this essay, we will explore the intricacies of oxidation and its far-reaching implications on our planet and our daily lives.At its core, oxidation is a chemical reaction in which a substance loses electrons, resulting in an increase in its oxidation state. This process is ubiquitous in nature, occurring in everything from the rusting of metal to the cellular respiration that sustains life. The importance of oxidation cannot be overstated as it is integral to numerous essential processes that sustain our world.One of the most well-known examples of oxidation is the rusting of iron. When iron is exposed to air and moisture, it undergoes a series of chemical reactions that cause it to slowly deteriorate and transform into a reddish-brown substance known as iron oxide. Thisprocess not only affects the structural integrity of the metal but also has significant implications for industries that rely on iron-based materials. Engineers and architects must account for the effects of oxidation when designing structures, vehicles, and infrastructure to ensure their longevity and safety.Beyond the realm of industry, oxidation also plays a crucial role in the natural world. In the atmosphere, the process of oxidation is responsible for the formation of ozone, a gas that shields the Earth from the harmful effects of ultraviolet radiation. Ozone, created through the interaction of oxygen molecules and solar energy, acts as a protective layer, filtering out these damaging rays and maintaining a habitable environment for life on our planet.Oxidation also lies at the heart of the carbon cycle, a fundamental process that regulates the exchange of carbon between the Earth's various systems, including the atmosphere, biosphere, and lithosphere. Through the process of photosynthesis, plants absorb carbon dioxide from the air and, using the energy from the sun, convert it into organic compounds, releasing oxygen in the process. This oxygen is then utilized by living organisms, including humans, in the process of cellular respiration, where it is combined with glucose to produce energy. The carbon, in turn, is released back into the atmosphere as carbon dioxide, completing the cycle.The delicate balance of this cycle is crucial for maintaining the Earth's atmospheric composition and climate. Disruptions to the carbon cycle, often caused by human activities such as the burning of fossil fuels, can lead to an imbalance in the levels of greenhouse gases, contributing to climate change and its far-reaching consequences.Oxidation also plays a crucial role in the human body, where it is responsible for the generation of energy through the process of cellular respiration. In this process, oxygen is used to break down glucose and other organic compounds, releasing the energy stored within these molecules. This energy is then used to power the various functions of the body, from the beating of the heart to the firing of neurons in the brain.However, the process of oxidation can also have negative consequences for the human body. Excessive oxidation, known as oxidative stress, can lead to the formation of harmful free radicals, which can damage cells and contribute to the development of various diseases, including cancer, heart disease, and neurodegenerative disorders. To combat this, the body has developed a complex system of antioxidants, which work to neutralize these free radicals and maintain a healthy balance of oxidation within the cells.The importance of oxidation extends far beyond the realms ofindustry, the natural world, and human health. In the field of energy production, oxidation plays a crucial role in the generation of electricity and the powering of various technologies. The burning of fossil fuels, for example, is a process of oxidation that releases the energy stored within these compounds, which can then be harnessed to generate electricity and power our homes, businesses, and transportation systems.Similarly, the development of renewable energy sources, such as solar and wind power, also relies on the principles of oxidation. In the case of solar power, the interaction between photons of light and the electrons in solar cells leads to the generation of an electrical current, a process that is fundamentally driven by the principles of oxidation and reduction.As we look to the future, the understanding and management of oxidation will become increasingly crucial in addressing the pressing challenges facing our world. From the development of more efficient and sustainable energy sources to the design of materials that are resistant to corrosion, the ability to harness and control the power of oxidation will be key to ensuring a brighter and more sustainable future for all.In conclusion, the oxidation situation is a complex and multifaceted phenomenon that permeates every aspect of our lives, from thenatural world to the technological innovations that power our societies. By deepening our understanding of this fundamental chemical process, we can unlock new possibilities for addressing the challenges of our time and shaping a better tomorrow for generations to come.。
胡萝卜益处英文作文

胡萝卜益处英文作文The Numerous Benefits of Carrots.Carrots, often described as nature's little sticks of sweetness, are a root vegetable that not only enhances the flavor of dishes but also packs a powerful punch of nutrients. Their bright orange hue is a testament to their rich beta-carotene content, which converts into vitamin A in the body, essential for maintaining good vision and overall health. However, the benefits of carrots extend far beyond their visual appeal and nutritional value.Enhanced Vision.Carrots are widely known for their role in promoting healthy vision. The beta-carotene they contain convertsinto vitamin A in the body, which is crucial for maintaining the health of the retina, the light-sensitive tissue that lines the inner eye. Vitamin A deficiency can lead to conditions such as night blindness, where the eyeshave difficulty adapting to low-light environments. By consuming carrots regularly, individuals can help prevent these vision-related issues.Boosted Immune System.Carrots are also rich in beta-cryptoxanthin, another carotenoid that acts as an antioxidant in the body. Antioxidants help neutralize harmful free radicals, which can damage cells and lead to chronic diseases. Byprotecting cells from oxidative stress, beta-cryptoxanthin and other antioxidants in carrots support a healthy immune system, reducing the risk of infections and chronic inflammatory conditions.Improved Digestion.Carrots are high in fiber, which is essential for maintaining a healthy digestive system. Fiber adds bulk to the stool, helping it move through the intestines more smoothly. This prevents conditions such as constipation and promotes the growth of healthy bacteria in the gut.Furthermore, carrots contain water and enzymes that aid in digestion, making them an excellent addition to a balanced diet.Weight Management.Carrots are low in calories but high in fiber and water content, making them an ideal snack for weight management. Eating carrots can help individuals feel full without adding significant calories to their diet. Additionally, the fiber in carrots helps slow down digestion, preventing spikes in blood sugar levels, which is beneficial for weight control and overall health.Heart Health.The potassium and folate found in carrots play acrucial role in maintaining heart health. Potassium helps regulate blood pressure by counteracting the effects of sodium, while folate is essential for the metabolism of homocysteine, an amino acid that can contribute to heart disease when present in high levels. By consuming carrots,individuals can help support a healthy cardiovascular system.Cancer Prevention.Carrots contain phytochemicals, compounds that have been shown to have anticancer properties. In particular, the beta-carotene and other carotenoids in carrots have been studied for their potential to prevent certain types of cancer, including lung cancer and skin cancer. While the exact mechanism is still being studied, it is believed that these compounds may help protect cells from damage that can lead to cancer development.Improved Skin Health.The vitamin A in carrots also plays a role in maintaining healthy skin. Vitamin A is essential for skin cell turnover, helping to keep the skin looking smooth and youthful. Additionally, the antioxidants in carrots help protect the skin from damage caused by environmentalfactors such as UV radiation and pollution.Conclusion.In conclusion, carrots are not just a tasty addition to meals; they are a nutritional powerhouse that offers a wide range of health benefits. From promoting healthy vision and a strong immune system to supporting weight management and heart health, carrots are a versatile vegetable that should be included in everyone's diet. Whether eaten raw as a snack, grated into salads, or cooked into delicious dishes, carrots are a delicious way to improve one's overall health and well-being.。
健脑食品的好处英文作文

健脑食品的好处英文作文Nourishing the Brain: The Remarkable Benefits of Brain-Boosting FoodsThe human brain is a remarkable organ, responsible for our cognitive abilities, emotions, and the very essence of who we are. As such, it is crucial that we provide our brains with the proper nourishment to function at their optimal level. Fortunately, there are a variety of foods that have been shown to have a positive impact on brain health and cognitive function. In this essay, we will explore the remarkable benefits of incorporating brain-boosting foods into our diets.One of the most well-known brain-boosting foods is the humble blueberry. These small, dark-colored berries are packed with antioxidants, which play a crucial role in protecting the brain from the damaging effects of oxidative stress. Studies have shown that regular consumption of blueberries can improve memory, cognitive function, and even reverse age-related declines in brain function. The antioxidants found in blueberries, known as anthocyanins, have beenfound to enhance the communication between brain cells, improving overall brain health.Another powerful brain-boosting food is the omega-3 fatty acid-rich fish, such as salmon, mackerel, and sardines. Omega-3 fatty acids are essential for the proper development and function of the brain, as they are a key component of the cell membranes that surround brain cells. Numerous studies have demonstrated that individuals who consume more omega-3 fatty acids have a reduced risk of developing cognitive decline, Alzheimer's disease, and other forms of dementia. Omega-3 fatty acids have also been shown to improve mood, reduce inflammation, and enhance learning and memory.Nuts and seeds are another category of brain-boosting foods that deserve attention. Walnuts, in particular, are a standout, as they are rich in a variety of nutrients that are essential for brain health, including omega-3 fatty acids, antioxidants, and the mineral copper. Walnuts have been shown to improve cognitive function, enhance memory, and even protect the brain from the damaging effects of stress. Other nuts and seeds, such as almonds, chia seeds, and flaxseeds, are also excellent sources of brain-boosting nutrients, including vitamin E, magnesium, and zinc.Leafy green vegetables, such as kale, spinach, and Swiss chard, are also highly beneficial for brain health. These nutrient-dense greensare rich in folate, a B vitamin that is essential for the proper development and function of the brain. Folate deficiency has been linked to an increased risk of cognitive decline and dementia, making leafy greens an essential component of a brain-healthy diet. Additionally, the antioxidants and anti-inflammatory compounds found in leafy greens can help protect the brain from the damaging effects of oxidative stress and inflammation.Turmeric, a vibrant yellow spice that is a staple in many Asian cuisines, is another brain-boosting food that deserves attention. The active compound in turmeric, known as curcumin, has been shown to have powerful anti-inflammatory and antioxidant properties that can protect the brain from the damaging effects of neuroinflammation and oxidative stress. Studies have also suggested that curcumin may enhance cognitive function, improve memory, and even reduce the risk of Alzheimer's disease.Finally, dark chocolate is a surprising addition to the list of brain-boosting foods. While it may be tempting to indulge in chocolate for its delicious taste, it also offers some remarkable benefits for brain health. Dark chocolate is rich in flavonoids, a type of antioxidant that has been shown to improve blood flow to the brain, enhance cognitive function, and even protect the brain from age-related decline. Additionally, dark chocolate contains small amounts of stimulants, such as caffeine and theobromine, which can temporarilyboost mood and cognitive performance.In conclusion, the foods we consume play a crucial role in the health and function of our brains. By incorporating a variety of brain-boosting foods, such as blueberries, salmon, walnuts, leafy greens, turmeric, and dark chocolate, into our diets, we can nourish our brains and reap the remarkable benefits of improved cognitive function, enhanced memory, and reduced risk of age-related cognitive decline. As we continue to uncover the powerful effects of these foods on brain health, it is clear that a well-balanced, nutrient-rich diet is essential for maintaining a healthy and vibrant brain throughout our lives.。
英语科普:物品有好闻的气味事出有因
Antioxidants are natural food ingredients that protect cells from harmful influences. Their main task is to neutralize1 so-called "free radicals2" which are produced in the process of oxidation and which are responsible for cell degeneration. Scientists at theMax Planck Institute for Chemical Ecology in Jena, Germany, and the University of Lund, Sweden, now show that vinegar flies are able to detect these protective substances by using olfactory3 cues. Odors that are exclusively derived4 from antioxidants attract flies, increase feeding behavior and trigger oviposition in female flies. (Current Biology, January 2015) Hydroxycinnamic acids are secondary plant metabolites and important dietary antioxidants. For animals as well as humans, antioxidants are essential components5 of a healthy diet, because they protect the cells and boost the immune system. Notably6, they prevent the emergence7 of too many free radicals, mostly oxygen compounds, and therefore a metabolic8 condition, which is generally called oxidative stress. If an organism suffers from oxidative stress, free radicals attack its cells and weaken its immune system. In fruit flies, oxidative stress is induced by immune responses to toxins9 produced by pathogens in the food.Hydroxycinnamic acids are found in high amounts in fruit. Since fruit is the preferred breeding substrate of fruit flies, scientists in the Department of Evolutionary10 Neuroethology at the Max-Planck-Institute for Chemical Ecology in Jena, Germany, took a closer look at these substances and their possible effect on the flies.Fruit flies are not able to smell hydroxycinnamic acids directly. However, yeasts12 metabolize the antioxidants and produce ethylphenols. These volatile13 substances activate14 targeted olfactory neurons housed on the maxillary palps of the fruit flies, which express the odorant receptor Or71a. Interestingly, fly larvae15 which are also attracted by yeasts enriched with hydroxycinnamic acids using ethylphenols as olfactory cues, employ another odorant receptor for binding16 ethylphenols: Or94b, which is exclusively found in larvae, and which is co-expressed with Or94a, a receptor binding a general yeast11 odor. Because flies cannot smell the antioxidants directly, ethylphenols provide reliable cues for the presence of these protective compounds in the food. The perception of these odorant signals has a direct impact of the flies' behavior: They are attracted by the odor sources, show increased feeding behavior and choose oviposition sites where ethylphenols indicate that antioxidants are present in the breeding substrate."This form of olfactory proxy17 detection is not only a phenomenon in insects. It has also been shown in humans, that odors that we perceive as pleasant or appetizing, are in fact derived from important and healthy nutrients18, such as essential amino acids, fatty acids and vitamins," Marcus Stensmyr explains. The scientist, who carried out the studies in the Department of Evolutionary Neuroethology together with his colleagues, has recently moved to a position as senior lecturer at the University of Lund.These findings demonstrate a further example of an individual neuronal pathway, which has a profound effect on the flies: from the odorant signal to olfactory neurons and dedicated19 odorant receptors to behavior. The ethylphenol pathway as anolfactory proxy detection of dietary antioxidants shows yet another facet20 of the complex odor-guided behavior in fruit flies. The scientists will now try to identify further neural21 pathways involved in the detection of essential nutrients, whichultimately trigger the flies' behavior.词汇解析表:1 neutralizev.使失效、抵消,使中和参考例句:Nothing could neutralize its good effects.没有什么能抵消它所产生的好影响。
诺伟司猪氧化应激管理及胴体品质
达到上市体重的猪的黄脂 Yellow Fat in Market Weight Pigs
高色素 HIGHLY PIGMENTED 正常 NORMAL
普通 MODERATE
脂褐质黄-褐色素导致黄脂,与细胞膜损伤有关 Lipofuscin yellow-brown pigment causes yellow appearance of fat, associated with membrane damage
Control 对照组
AOX
Other potential contributors to Yellow Fat
• Hepatotoxicity
• Obstructive , toxic/infective or hemolytic
Membrane Lipid Bilayer
过氧化物引发氧化 Peroxide initiates oxidation
=PUFA更易于氧化
= PUFA more subject to oxidation
•被氧化的膜上脂肪酸 Oxidized membrane FA’s • 细胞的溶酶体降解 Lysosomal degradation of cell • 吞噬细胞的渗入 Infiltration by phagocytes • 变性的脂肪细胞 Degenerated fat cells
后果 Consequences
分子损伤
H2O2 Major ROS .O .OH
Molecular Damage •脂类 Lipids •蛋白质 Proteins •核酸 Nucleic Acids
Infection: bacterial, parasite 肺性高血压 Pulmonary Hypertension 霉菌毒素 Mycotoxins
NOX介导的氧化应激对肝纤维化相关信号通路调控的研究进展
NOX介导的氧化应激对肝纤维化相关信号通路调控的研究进展张望;朱萱【摘要】肝纤维化是各种慢性肝脏疾病的共同病理结果,以细胞外基质尤其是Ⅰ型和Ⅲ型胶原的过度沉积为主要特点,其持续进展可导致肝硬化,甚至肝癌.NADPH氧化酶(NOX)是一种多亚基组成的跨膜酶复合物,众多研究表明其介导的氧化应激在肝纤维化的发病机制中发挥重要作用,并参与调控多条肝纤维化相关信号通路,如TGF-β/Smad信号通路、MAPK信号通路、PI3 K-AKT信号通路、NF-κB信号通路.【期刊名称】《山东医药》【年(卷),期】2017(057)009【总页数】4页(P100-103)【关键词】NADPH氧化酶;氧化应激;肝纤维化;信号通路【作者】张望;朱萱【作者单位】南昌大学第一附属医院,南昌330006;南昌大学第一附属医院,南昌330006【正文语种】中文【中图分类】R575.2肝纤维化是肝脏对各种慢性损伤刺激发生修复反应的共同病理结果[1],其以细胞外基质(ECM)尤其是Ⅰ型和Ⅲ型胶原的过度沉积为主要特点,肌成纤维细胞是肝纤维化发生时ECM的主要来源,肝纤维化的持续进展可演变为肝硬化,甚至肝癌。
目前国内外大量研究表明肝星状细胞(HSCs)活化、增殖为肝纤维化发生的中心事件。
众多因素(如肝细胞凋亡、细胞因子刺激等)可导致HSCs活化,活化的HSCs形态和功能发生一系列改变,转变为肌成纤维细胞,导致ECM大量分泌,从而引起肝纤维化的发生。
越来越多的研究表明氧化应激与HSCs的活化、肝纤维化的发生密切相关,而NADPH氧化酶(NOX)作为目前唯一已知的专职产生活性氧簇(ROS)的酶类,其介导的氧化应激在肝纤维化发病中起重要作用,抑制NOX介导的氧化应激能明显减少HSCs活化,抑制肝纤维化发生。
现就NOX介导的氧化应激对肝纤维化相关信号通路调控的研究进展作一综述。
氧化应激是指机体反应活性氧簇(ROS)的产生与抗氧化防御系统(酶性抗氧化剂和非酶性抗氧化剂)之间的平衡被打破,机体ROS过度产生,内源性抗氧化防御系统功能减退,从而引起机体组织细胞发生损伤[2]。
胡萝卜的好处英文作文
胡萝卜的好处英文作文"英文,"Carrots are truly amazing vegetables that offer numerous health benefits. As a vegetable enthusiast, I can attest to their versatility and nutritional value.First and foremost, carrots are packed with essential nutrients such as vitamin A, vitamin C, potassium, and fiber. These nutrients play crucial roles in maintaining overall health. For instance, vitamin A is essential for good vision, and consuming carrots regularly can help prevent conditions like night blindness. Additionally, the fiber content in carrots aids in digestion and promotes a healthy gut.Moreover, carrots are known for their antioxidant properties, thanks to compounds like beta-carotene. Antioxidants help protect the body from oxidative stress and reduce the risk of chronic diseases such as heartdisease and certain types of cancer. As someone who values long-term health, incorporating carrots into my diet is ano-brainer.Another benefit of carrots is their contribution to weight management. Since they are low in calories and highin fiber, they can help keep you feeling full and satisfied, thus preventing overeating. As someone who enjoys maintaining a healthy weight without feeling deprived, carrots are a staple in my daily meals.Furthermore, carrots are incredibly versatile and canbe enjoyed in various ways. Whether raw, cooked, or juiced, there are countless delicious recipes that incorporate carrots. One of my favorite ways to enjoy them is in a classic carrot soup, especially on a chilly day. The sweetness of the carrots combined with savory spices makes for a comforting and nutritious meal.In addition to their nutritional value, carrots arealso budget-friendly and readily available year-round. Whether purchased fresh from the farmers' market or frozenfrom the grocery store, carrots are an affordable optionfor individuals and families looking to eat healthilywithout breaking the bank.In conclusion, the benefits of carrots are undeniable. From promoting good vision to supporting weight management and overall health, carrots are a true superfood. So next time you're planning your meals, don't forget to includethis humble yet mighty vegetable!"中文,"胡萝卜真是一种令人惊叹的蔬菜,提供了许多健康益处。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1360-1385/02/$ – see front matter © 2002 Elsevier Science Ltd. All rights reserved. PII: S1360-1385(02)02312-9Ron MittlerDept of Botany, Plant Sciences Institute,353Bessey Hall, Iowa State University, Ames,IA 50011, USA.e-mail: rmittler@Reactive oxygen intermediates (ROIs) are partially reduced forms of atmospheric oxygen (O 2). Theytypically result from the excitation of O 2to form singlet oxygen (O 21) or from the transfer of one, two or three electrons to O 2to form, respectively , a superoxide radical (O 2−), hydrogen peroxide (H 2O 2) or a hydroxyl radical (HO −). In contrast to atmospheric oxygen, ROIs are capable of unrestricted oxidation of various cellular components and can lead to the oxidative destruction of the cell [1–4].Production of ROIs in cellsThere are many potential sources of ROIs in plants (Table 1). Some are reactions involved in normalmetabolism, such as photosynthesis and respiration.These are in line with the traditional concept,considering ROIs as unavoidable byproducts ofaerobic metabolism [1]. Other sources of ROIs belong to pathways enhanced during abiotic stresses, such as glycolate oxidase in peroxisomes during photorespiration. However, in recent years, new sources of ROIs have been identified in plants,including NADPH oxidases, amine oxidases and cell-wall-bound peroxidases. These are tightlyregulated and participate in the production of ROIs during processes such as programmed cell death (PCD) and pathogen defense [2,4,5].Whereas, under normal growth conditions, the production of ROIs in cells is low (240 µM s −1O 2−and a steady-state level of 0.5 µM H 2O 2in chloroplasts) [6],many stresses that disrupt the cellular homeostasis of cells enhance the production of ROIs (240–720 µM s −1O 2−and a steady-state level of 5–15 µM H 2O 2) [6].These include drought stress and desiccation, salt stress, chilling, heat shock, heavy metals, ultravioletradiation, air pollutants such as ozone and SO 2,mechanical stress, nutrient deprivation, pathogen attack and high light stress [2,7–10]. The production of ROIs during these stresses results from pathways such as photorespiration, from the photosynthetic apparatus and from mitochondrial respiration. In addition, pathogens and wounding or environmental stresses (e.g. drought or osmotic stress) have been shown to trigger the active production of ROIs byNADPH oxidases [4,11–13]. The enhanced production of ROIs during stress can pose a threat to cells but it is also thought that ROIs act as signals for the activation of stress-response and defense pathways [9,14]. Thus, ROIs can be viewed as cellular indicators of stress and as secondary messengers involved in the stress-response signal transduction pathway .Although the steady-state level of ROIs can be used by plants to monitor their intracellular level of stress, this level has to be kept under tight control because over-accumulation of ROIs can result in cell death [1–4]. ROI-induced cell death can result from oxidative processes such as membrane lipidperoxidation, protein oxidation, enzyme inhibition and DNA and RNA damage (the traditional concept).Alternatively , enhanced levels of ROIs can activate a PCD pathway , as was recently demonstrated by the inhibition of oxidative stress (paraquat)-induced cell death in tobacco by anti-apoptotic genes [15].Because ROIs are toxic but also participate in signaling events, plant cells require at least two different mechanisms to regulate their intracellular ROI concentrations by scavenging of ROIs: one that will enable the fine modulation of low levels of ROIs for signaling purposes, and one that will enable the detoxification of excess ROIs, especially during stress.In addition, the types of ROIs produced and the balance between the steady-state levels of different ROIs can also be important. These are determined by theinterplay between different ROI-producing and ROI-scavenging mechanisms, and can change drastically depending upon the physiological condition of the plant and the integration of different environmental,developmental and biochemical stimuli.Scavenging of ROIs in cellsMajor ROI-scavenging mechanisms of plants include superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT) [1,7,16] (Table 1). The balance between SOD and APX or CAT activities in cells is crucial for determining the steady-state level ofT raditionally,reactive oxygen intermediates (ROIs) were considered to be toxic by-products of aerobic metabolism,which were disposed of using antioxidants.However,in recent years,it has become apparent that plants actively produce ROIs as signaling molecules to control processes such as programmed cell death,abiotic stress responses,pathogen defense and systemic signaling.Recent advances including microarray studies and the development of mutants with altered ROI-scavenging mechanisms provide new insights into how the steady-state level of ROIs are controlled in cells.In addition,key steps of the signal transduction pathway that senses ROIs in plants have been identified.These raise several intriguing questions about the relationships between ROI signaling,ROI stress and the production and scavenging of ROIs in the different cellular compartments.Published online: 12 August 2002Oxidative stress,antioxidants and stress toleranceRon Mittlersuperoxide radicals and hydrogen peroxide [17]. This balance, together with sequestering of metal ions, is thought to be important to prevent the formation of the highly toxic hydroxyl radical via the metal-dependent Haber–Weiss or the Fenton reactions [1]. The different affinities of APX (µM range) and CAT (m M range) for H 2O 2suggest that they belong to two differentclasses of H 2O 2responsible for the fine modulation of ROIs forsignaling, whereas CAT might be responsible for or the removal of excess ROIs during stress.The major ROI-scavenging pathways of plants (Fig. 1) include SOD, found in almost all cellular compartments, the water–water cycle inchloroplasts (Fig. 1a), the ascorbate–glutathione cycle in chloroplasts, cytosol, mitochondria, apoplast and peroxisomes (Fig. 1b), glutathione peroxidase (GPX; Fig. 1c), and CAT in peroxisomes (Fig. 1d). The finding of the ascorbate–glutathione cycle in almost all cellular compartments tested to date, as well as the high affinity of APX for H 2O 2, suggests that this cycle plays a crucial role in controlling the level ofROIs in these compartments. By contrast, CAT is only present in peroxisomes, but it is indispensable for ROI detoxification during stress, when high levels of ROIs are produced [16]. In addition, oxidative stress causes the proliferation of peroxisomes [18]. Drawingupon the model for bacteria [19], a dense population of peroxisomes might be highly efficient in scavenging of ROIs, especially H 2O 2, which diffuses into peroxisomes from the cytosol.The water–water cycle (Fig. 1a) draws its reducing energy directly from the photosynthetic apparatus [3]. Thus, this cycle appears to be autonomous with respect to its energy supply . However, the source of reducing energy for ROI scavenging by theascorbate–glutathione cycle (Fig. 1b) during normal metabolism and particularly during stress, when the photosynthetic apparatus might be suppressed or damaged, is not entirely clear. In animals and yeast,the pentose-phosphate pathway is the main source of NADPH for ROI removal [20,21]. Because CAT does not require a supply of reducing equivalents for its function, it might be insensitive to the redox status of cells and its function might not be affected during stress, unlike the other mechanisms (Fig. 1).Antioxidants such as ascorbic acid and glutathione,which are found at high concentrations in chloroplasts and other cellular compartments (5–20 m M ascorbic acid and 1–5 m M glutathione) are crucial for plant defense against oxidative stress [8]. Consequently ,both mutants with suppressed ascorbic acid levels [22] and transgenic plants with altered content of glutathione [23] are hypersensitive to stress conditions. It is generally believed that maintaining a high reduced per oxidized ratio of ascorbic acid and glutathione is essential for the proper scavenging of ROIs in cells. This ratio is maintained by glutathione reductase (GR),monodehydroascorbate reductase (MDAR) anddehydroascorbate reductase (DHAR) using NADPH as reducing power (Fig. 1) [3,8]. In addition, the overall balance between different antioxidants has to betightly controlled. Enhanced glutathione biosynthesis in chloroplasts can result in oxidative damage to cells rather than their protection, possibly by altering the overall redox state of chloroplasts [23]. It has also been suggested that the oxidized:reduced ratio of the different antioxidants can serve as a signal for the modulation of ROI-scavenging mechanisms [24].Avoiding ROI productionAvoiding ROI production might be as important as active scavenging of ROIs. Because many abiotic stress conditions are accompanied by an enhanced rate of ROI production, avoiding or alleviating the effects of stresses such as drought or high light on plant metabolism will reduce the risk of ROI production. Mechanisms that might reduce ROI production during stress (Table 1) include:(1)anatomical adaptations such as leaf movement and curling, development of a refracting epidermis and hiding of stomata in specialized structures;(2)physiological adaptations such as C 4and CAM metabolism; and (3) molecular mechanisms that rearrange the photosynthetic apparatus and its antennae in accordance with light quality andintensity or completely suppress photosynthesis. Bybalancing the amount of light energy absorbed by the plant with the availability of CO 2, these mechanisms might represent an attempt to avoid the over-reduction of the photosynthetic apparatus and the transfer of electrons to O 2rather than for CO 2fixation.ROI production can also be decreased by the alternative channeling of electrons in the electron-transport chains of the chloroplasts and mitochondria by a group of enzymes called alternative oxidases (AOXs). AOXs can divert electrons flowing through electron-transport chains and use them to reduce O 2to water (Fig. 2). Thus, they decrease ROI production by two mechanisms: they prevent electrons fromreducing O 2into O 2−and they reduce the overall level of O 2, the substrate for ROI production, in theorganelle. Decreasing the amount of mitochondrial AOX increases the sensitivity of plants to oxidative stress [25]. In addition, chloroplast AOX is induced in transgenic plants that lack APX and/or CAT, and in normal plants in response to high light [50].Production and scavenging of ROIs in different cellular compartmentsRecent manipulations of ROI-scavenging pathways in different cellular compartments suggest someintriguing possibilities. For years, the chloroplast was considered to be the main source of ROI production in cells and consequently one of the main targets for ROI damage during stress. However, it has recently been suggested that the chloroplast is not as sensitive to ROI damage as previously thought [26]. The mitochondrion is another cellular site of ROI production. However,recent studies suggest that the mitochondrion is also a key regulator of PCD in plants and that enhanced ROIs levels at the mitochondria can trigger PCD [27].Both the mitochondrion and the chloroplast contain ROI-scavenging mechanisms. By contrast, little is known about the ROI-scavenging properties of the nucleus, which might contain redox-sensitivetranscription factors [28]. Because H 2O 2can diffuse through aquaporins [29], ROIs produced at a specific cellular site (e.g. the chloroplast during stress or the apoplast during pathogen attack) can affect other cellular compartments, overwhelm theirROI-scavenging capabilities and alter the pattern of gene expression during stress, pathogen infection or PCD. In support of this assumption, stresses that result in the enhanced production of ROIs at thechloroplast induce cytosolic and not chloroplastic ROI-scavenging mechanisms [24,30], and ROI production at the apoplast induces the production of pathogenesis-response proteins [4]. Because the plant mitochondria and nuclei are involved in the activation of PCD [27],the level of ROIs that reaches these compartments during stress or pathogen challenge needs to be tightly controlled to prevent abnormal PCD activation. The cytosol, with its ascorbate–glutathione cycle, and the peroxisomes, with CAT, might therefore act as a buffer zone to control the overall level of ROIs that reaches different cellular compartments during stress and normal metabolism.The importance of peroxisomes in ROI metabolism is beginning to gain recognition [31]. Peroxisomes are not only the site of ROI detoxification by CAT but also the site of ROI production by glycolate oxidase and fatty acid β-oxidation. In addition, peroxisomes might be one of the cellular sites for nitric oxide (NO)biosynthesis [31]. In animal cells, NO activates fatty acid β-oxidation and enhances the production of ROIs in cells. However, although NO has been shown to be involved in ROI-induced cell death in plants [32] and NO is known to be a key regulator of pathogen responses [5], little is known about how NO isinvolved in the response of plants to abiotic stresses.Redundancy in ROI-scavenging mechanismsSome of the complex relationships between the different ROI-scavenging and ROI-producingmechanisms have been revealed in transgenic plants with suppressed production of ROI-detoxifying mechanisms. Thus, plants with suppressed APXFig. 1.Pathways for reactive oxygen intermediate (ROI)scavenging in plants.(a)The water –water cycle.(b) The ascorbate –glutathione cycle. (c) The glutathione peroxidase (GPX) cycle. (d) Catalase (CAT). Superoxidedismutase (SOD) acts as the first line of defense converting O 2−into H 2O 2.Ascorbate peroxidases (APX), GPX and CAT then detoxify H 2O 2. In contrast to CAT (d), APX and GPX require an ascorbate (AsA)and/or a glutathione (GSH)regenerating cycle (a –c).This cycle uses electrons directly from thephotosynthetic apparatus (a) or NAD(P)H (b,c) as reducing power. ROIs are indicated in red,antioxidants in blue and ROI-scavenging enzymes in green. Abbreviations:DHA, dehydroascorbate;DHAR, DHA reductase;Fd,ferredoxin; GR,glutathione reductase;GSSG, oxidized glutathione; MDA,monodehydroascorbate;MDAR, MDA reductase;PSI,photosystem I; tAPX,thylakoid-bound APX.production induce SOD, CAT and GR to compensate for the loss of APX, whereas plants with suppressed CAT production induce APX, GPX and mitochondrial AOX [16,50]. CAT and APX are not completely redundant because they do not compensate for the lack of each other, as shown by the sensitivity of plantswith reduced APX or CAT levels to environmental stresses and pathogen attack [33]. Interestingly ,plants with suppressed APX and CAT appeared, at least under a defined set of environmental conditions,to be less sensitive to oxidative stress than plants with lowered APX or CAT levels. These plants had reduced photosynthetic activity , enhanced chloroplastic AOX production and enhanced expression of genes of the oxidative and reductive pentose-phosphate pathway and MDAR, possibly to avoid ROI production as well as to enhance the non-enzymatic detoxification of H 2O 2by ascorbic acid [50].ROIs at the interface between biotic and abiotic stressesROIs play a central role in the defense of plants against pathogen attack. During this response, ROIs are produced by plant cells via the enhanced enzymatic activity of plasma-membrane-bound NADPH oxidases,cell-wall-bound peroxidases and amine oxidases in the apoplast [4,5]. H 2O 2produced during this response (up to 15 µM ; directly or as a result of superoxide dismutation) is thought to diffuse into cells and,together with salicylic acid (SA) and NO [34], toactivate many of the plant defenses, including PCD [35].The activity of APX and CAT is suppressed during this response by the plant hormones SA and NO [34], the production of APX is post-transcriptionally suppressed [36] and the production of CAT is downregulated at the level of steady-state mRNA [37]. Thus, the plantsimultaneously produces more ROIs and at the same time diminishes its own capacity to scavenge H 2O 2,resulting in the over-accumulation of ROIs and the activation of PCD. The suppression of ROI-scavenging mechanisms together with the synthesis of NO appears to be crucial for the activation of PCD because, in their absence, increased ROI production at the apoplast does not result in the induction of PCD [32,33].The role ROIs play during PCD appears, therefore,to be opposite to the role they play during abiotic stresses, during which ROIs induce ROI-scavenging mechanisms such as APX and CAT that decrease the steady-state level of ROIs in cells (Fig. 3). The differences in the function of ROIs between biotic and abiotic stresses might result from the action of hormones such as SA and NO, from cross-talkbetween different signaling pathways (Fig. 4) or from differences in the steady-state level of ROIs produced during the different stresses. The apparent conflict in ROI metabolism between biotic and abioticstresses (Fig. 3) raises the question of how the plant manipulates its rate of ROI production and ROIscavenging when it comes under biotic attack during an abiotic stress. In support of the possible existence of such a conflict, tobacco plants that were previously subjected to oxidative stress (and consequently had a higher level of antioxidative enzymes) had a reduced rate of PCD compared with unstressed control plants [33]. In addition, plants that overproduce CAT have a decreased resistance to pathogen infection [38].Fig. 2.Involvement of alternative oxidase (AOX) in reactive oxygen intermediate (ROI) avoidance.In both the mitochondrial electron-transport chain (a) and the chloroplast electron-transport chain (b),AOX diverts electrons that can be used to reduce O 2into O 2−and uses these electrons to reduce O 2to H 2O. In addition, AOX reduces the overall level of O 2, the substrate for ROI production, in theorganelle. AOX is indicated in yellow and the different components of the electron-transport chain are indicated in red, green or gray. Abbreviations: Cyt-b 6f , cytochrome b 6f ; Cyt-c, cytochrome c ;Fd,ferredoxin; PC, plastocyanin; PSI, PSII, photosystems I and II.Fig. 3.Differences in the steady-state levels of reactive oxygen intermediates (ROI) during biotic stress and abiotic stress. Biotic stress (a) results in the activation of NADPH oxidase and the suppression of ascorbate peroxidase (APX) and catalase (CAT). This leads to the over-accumulation of ROI and the activation of defense mechanisms. Abiotic stress (b) enhances ROI production by chloroplasts and mitochondria. However, by inducing ROI-scavenging enzymes such as APX and CAT , it reduces ROI levels. The question mark indicates that little is known about the regulation of ROI metabolism during a combination of biotic and abiotic stresses. Chloroplasts are indicated in green, mitochondria in gray and the steady-state levels of ROI in yellow.ROI signal transduction pathwayRecent studies have identified several components involved in the signal transduction pathway of plants that senses ROIs. These include the mitogen-activated protein (MAP) kinase kinase kinases AtANP1 and NtNPK1, and the MAP kinases AtMPK3/6 and Ntp46MAPK [39,40]. In addition, calmodulin has been implicated in ROI signaling [9,41]. A hypothetical model depicting some of the players involved in this pathway is shown in Fig. 4. H 2O 2is sensed by a sensor that might be a two-component histidine kinase, as in yeast [9]. Calmodulin and a MAP-kinasecascade are then activated, resulting in the activation or suppression of several transcription factors. These regulate the response of plants to oxidative stress [9,42]. Cross-talk with the pathogen-response signal transduction pathway also occurs and might involve interactions between different MAP-kinase pathways,feedback loops and the action of NO and SA as key hormonal regulators. This model (Fig. 4) is simplified and is likely to change as research advances our understanding of this pathway .ROIs act as signals that mediate the systemic activation of gene expression in response to pathogenpathway by ROIs? It is possible that the level of H 2O 2that is currently thought to kill cells by direct cellular damage actually induces PCD [15,27], and it might require a higher level of ROIs to kill cells by direct oxidation. Perhaps future studies applying oxidative stress to mutants deficient in different PCD pathways will answer this question.Many questions related to ROI metabolism remain unanswered (Box 1). We are currently at an exciting time, when most of the technologies required to answer these questions are in place. Thus, acomprehensive analysis of gene expression using microarrays and chips, coupled with proteomics andAcknowledgementsI apologize to all colleagues whose work could not be reviewed here because of space limitation. I thank Barbara A.Zilinskas and Eve Syrkin Wurtele for critical reading of the manuscript. Research at my laboratory is supported by funding from the Israeli Academy of Sciences and the Biotechnology Council of Iowa State University.Fig. 4.A suggested model for the activation of signal transduction events during oxidative stress.H 2O 2is detected by a cellular receptor or sensor. Its detection results in the activation of a mitogen-activated-protein kinase (MAPK) cascade and a group of transcription factors that control different cellular pathways. H 2O 2sensing is also linked to changes in the levels of Ca 2+and calmodulin, and to the activation or induction of a Ca 2+–calmodulin kinase that can also activate or suppress the activity of transcription factors. The regulation of gene expression by the different transcription factors results in the induction of various defense pathways, such as reactive oxygen intermediate (ROI) scavenging and heat-shock proteins (HSPs), and in the suppression of some ROI-producing mechanisms and photosynthesis. There is also cross-talk with the plant –pathogen signal transduction pathway, which might depend on pathogen recognition by the gene-for-gene mechanism and can result in an inverse effect on the regulation of ROI-production and ROI-scavenging mechanisms, as well as on theactivation of programmed cell death (PCD). The plant hormones nitric oxide (NO) and salicylic acid (SA) are key regulators of this response.metabolomics to follow different antioxidants and related compounds during oxidative stress, should answer many of these questions. This analysis can be performed on plants responding to abiotic stresses,biotic insults or combinations of both, and can be complemented by using mutants with altered abilityto produce or scavenge ROIs. In addition, thedevelopment of cellular markers that enable the non-destructive quantification of different ROIs in the different cellular compartments, like the markers used for Ca 2+imaging, will considerably advance our understanding of ROI metabolism.References1Asada, K. and Takahashi, M. (1987) Production and scavenging of active oxygen in photosynthesis. In Photoinhibition (Kyle, D.J. et al., eds), pp.227–287,Elsevier2Dat, J. et al.(2000) Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci.57, 779–7953Asada, K. (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol.50, 601–6394Hammond-Kosack, K.E. and Jones, J.D.G. (1996)Resistance gene-dependent plant defense responses. Plant Cell 8, 1773–17915Grant, J.J. and Loake, G.J. (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol.124, 21–296Polle, A. (2001) Dissecting the superoxidedismutase–ascorbate peroxidase–glutathione pathway in chloroplasts by metabolic puter simulations as a step towards flux analysis. Plant Physiol.126, 445–4627Bowler, C. et al.(1992) Superoxide dismutases and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol.43, 83–1168Noctor, G. and Foyer, C. (1998) Ascorbate and glutathione: keeping active oxygen under control.Annu. Rev. Plant Physiol. Plant Mol. Biol.49,249–2799Desikin, R. et al.(2001) Regulation of theArabidopsis transcriptosome by oxidative stress.Plant Physiol.127, 159–17210Allen, R. (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol.107, 1049–105411Orozco-Cardenas, M. and Ryan, C.A. (1999)Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway . Proc. Natl. Acad. Sci.U.S.A.96, 6553–655712Cazale, A.C. et al.(1999) MAP kinase activation by hypoosmotic stress of tobacco cell suspensions:towards the oxidative burst response? Plant J.19,297–30713Pei, Z-M. et al.(2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406, 731–73414Knight, H. and Knight, M.R. (2001) Abiotic stress signalling pathways: specificity and cross-talk.Trends Plant Sci.6, 262–26715Mitsuhara, I. et al.(1999) Animal cell-deathsuppressors Bcl-x L and Ced-9 inhibit cell death in tobacco plants. Curr . Biol.9, 775–77816Willekens, H. et al.(1997) Catalase is a sink for H 2O 2and is indispensable for stress defence in C-3plants. EMBO J.16, 4806–481617Bowler, C. et al.(1991) Manganese superoxidedismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J.10,1723–173218Lopez-Huertas, E. et al.(2000) Stress induces peroxisome biogenesis genes. EMBO J.19,6770–677719Ma, M. and Eaton, J.W . (1992) Multicellular oxidant defense in unicellular organisms. Proc.Natl. Acad. Sci. U. S. A.89, 7924–792820Pandolfi, P .P . et al.(1995) Targeted disruption ofthe housekeeping gene encoding glucose6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J.14,5209–521521Juhnke, H. et al.(1996) Mutants that showincreased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Mol.Gen. Genet.252, 456–46422Conklin, P .L. et al.(1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. U.S.A.93,9970–997423Creissen, G. et al.(1999) Elevated glutathionebiosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11,1277–129224Karpinski, S. et al.(1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9, 624–64025Maxwell, D.P . et al.(1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. U. S. A.96,8271–827626Karpinska, B. et al.(2000) Antagonistic effects of hydrogen peroxide and glutathione on acclimation to excess excitation energy in Arabidopsis.IUBMB Life 50, 21–2627Lam, E. et al.(2001) Programmed cell death,mitochondria and the plant hypersensitive response. Nature 411, 848–85328Delauney , A. et al.(2000) H 2O 2sensing through oxidation of the Y ap1 transcription factor. EMBO J.19, 5157–516629Henzier, T . and Steudle, E. (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina : model calculations andmeasurements with the pressure probe suggest transport of H 2O 2across water channels .J. Exp.Bot.51, 2053–206630Y oshimura, K. et al.(2000) Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol.123, 223–23431Corpas, F .J. et al.(2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci.6,145–15032Delledonne, M. et al.(2001) Signal interactions between nitric oxide and reactive oxygenintermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. U. S. A.98, 13454–1345933Mittler, R. et al.(1999) Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyper-responsive to pathogen infection. Proc. Natl. Acad. Sci. U. S. A.96,14165–1417034Klessig, D.F . et al.(2000) Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad.Sci. U. S. A.97, 8849–885535Dangl, J.L. et al.(1996) Death don’t have no mercy:cell death programs in plant–microbe interactions.Plant Cell 8, 1793–180736Mittler, R. et al.(1998) Post-transcriptional suppression of cytosolic ascorbate peroxidaseexpression during pathogen-induced programmed cell death in tobacco. Plant Cell 10,461–47437Dorey , S. et al.(1998) T obacco class I and class II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol. Plant–Microbe Interact.11, 1102–110938Polidoros, A.N. et al.(2001) Transgenic tobaccoplants expressing the maize Cat2gene have altered catalase levels that affect plant–pathogen interactions and resistance to oxidative stress.Transgenic Res.10, 555–56939Kovtun, Y . et al.(2000) Functional analysis of oxidative stress-activated mitogen-activatedprotein kinase cascade in plants. Proc. Natl. Acad.Sci. U. S. A.97, 2940–294540Samuel, M.A. et al.(2000) Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J.22, 367–37641Harding, S.A. et al.(1997) Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species.EMBO J.16, 1137–114442Maleck, K. et al.(2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet.26, 403–41043Alvarez, M.E. et al.(1998) Reactive oxygenintermediates mediate a systemic signal network in the establishment of plant immunity . Cell 92,773–78444Mullineaux, P . and Karpinski, S. (2002) Signaltransduction in response to excess light: getting out of the chloroplast. Curr . Opin. Plant Biol.5,43–4845Zhang, S. and Klessig, D.F . (2001) MAPK cascades in plant defense signaling. Trends Plant Sci.6,520–52746Allan, A.C. and Fluhr, R. (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–157247Dixon, D.P . et al.(1998) Glutathione-mediated detoxification systems in plants. Curr . Opin. Plant Biol.1, 258–26648Baier, M. and Dietz, K.J. (1996) Primary structure and expression of plant homologues of animal and fungal thioredoxin-dependent peroxide reductases and bacterial alkyl hydroperoxide reductases. Plant Mol. Biol.31, 553–56449Mittler, R. et al.(2001) Living under a ‘dormant’canopy: a molecular acclimation mechanism of the desert plant Retama raetam.Plant J.25,407–41650Rizhsky , L. et al . Double antisense plantswith suppressed expression of ascorbate peroxidase and catalase are less sensitiveto oxidative stress than single antisense plants with suppressed expression ofascorbate peroxidase or catalase. Plant J . (in press)。
