YAMAWA 最新版中文型录
水害雑录:新字新仮名

もう畳を上げた方がよいでしょう、と妻や大きい子供らは騒ぐ。牛舎へも水が入りましたと若《わか》い衆《しゅ》も訴えて来た。
(数字は、JIS X 0213の面区点番号、または底本のページと行数)
(例)※[#「奚+隹」、第3水準1-93-66]が鳴く
-------------------------------------------------------
一
臆病者というのは、勇気の無い奴《やつ》に限るものと思っておったのは誤りであった。人間は無事をこいねがうの念の強ければ、その強いだけそれだけ臆病になるものである。人間は誰とて無事をこいねがうの念の無いものは無い筈であるが、身に多くの係累者を持った者、殊に手足まといの幼少者などある身には、更に痛切に無事を願うの念が強いのである。
五寸|角《かく》の土台数十丁一寸|厚《あつ》みの松板《まついた》数十枚は時を移さず、牛舎に運ばれた。もちろん大工を呼ぶ暇は無い。三人の男共を指揮して、数時間豪雨の音も忘れるまで活動した結果、牛舎には床上《ゆかうえ》更に五寸の仮床《かりゆか》を造り得た。かくて二十頭の牛は水上五寸の架床《かしょう》上に争うて安臥《あんが》するのであった。燃材《ねんざい》の始末、飼料品の片づけ、為すべき仕事は無際限にあった。
人間に対する用意は、まず畳を上げて、襖《ふすま》障子《しょうじ》諸財一切《しょざいいっさい》の始末を、先年《せんねん》大水《おおみず》の標準によって、処理し終った。並《なみ》の席より尺余《しゃくよ》床《ゆか》を高くして置いた一室と離屋《はなれ》の茶室の一間とに、家族十人の者は二分《にぶん》して寝に就く事になった。幼ないもの共は茶室へ寝るのを非常に悦んだ。そうして間もなく無心に眠ってしまった。二人の姉共と彼らの母とは、この気味の悪い雨の夜に別れ別れに寝るのは心細いというて、雨を冒《おか》し水を渡って茶室へやって来た。
67+4个Wii+Wiiware中文游戏全集(官中+汉化)

0----------LIST.TXT0----------更多游戏免费下载,请访问【w去ww掉.ggfans汉.n字et】01 - [R7GJAF]龙珠天下第一大冒险[猫星汉化][日版汉化]02 - [R8AJ01]宝可梦公园Wii 皮卡丘的大冒险[猫星汉化]03 - [R8DJA4]游戏王5D's:决斗狂热者[猫星汉化][日版汉化]04 - [R8EJQC]大地探索者[蓝宇汉化菜单][日版汉化]05 - [R8FJHA]快餐危机匠餐厅大繁盛![猫星汉化][日版汉化]06 - [R8PC01]超级纸片马里奥[ACG汉化]07 - [R49J01]大金刚丛林敲击[猫星汉化][日版汉化]08 - [R96JAF]风之克罗诺亚幻影之门[TGB汉化][日版汉化]09 - [RB2J2K]彩虹泡泡[猫星汉化][日版汉化]10 - [RBHJ08]生化危机0[xjsxjs197汉化][1 .2][日版汉化]11 - [RBUE08]生化危机安布雷拉历代记[猫星汉化][美版汉化]12 - [RCOC99]名侦探柯南追忆的幻想[ACG汉化]13 - [RD4JA4]劲舞革命劲爆舞会2[跳舞毯][猫星汉化][日版汉化]14 - [RDZJ01]天灾危机之日[猫星汉化][日版汉化]15 - [RE4J08]生化危机1[xjsxjs197汉化][2 .0][日版汉化]16 - [REKJ2N]有氧拳击 Wii快乐瘦身[猫星汉化][平衡板][日版汉化]17 - [REVJ8P]忌火起草解明篇[PLAY汉化公测版][日版汉化]18 - [RFEJ01]火焰纹章晓之女神[ACG汉化[V2][日版汉化]19 - [RFNW01]Wii健身[平衡板][台版官中]20 - [RFPW01]Wii健身加强版[平衡板][台版官中]21 - [RGSJ8P]幽灵小队[幽灵小队汉化][日版汉化]22 - [RHAW01]Wii第一次接触[台版官中]23 - [RHHJ8J]凉宫春日的激动[猫星汉化][日版汉化]24 - [RJOJJ9]恐怖体感咒怨[ACG汉化][日版汉化]25 - [RK5J01]星之卡比毛线传说[可伸缩汉化][日版汉化]26 - [RKZJA4]迷失蔚蓝幸存少年Wii[猫星汉化][日版汉化](含非日版机修复文件)27 - [RMCC01]马里奥赛车Wii[ACG汉化]28 - [RMGJ01]超级马里奥银河[ACG汉化][日版汉化]29 - [RMHJ08]怪物猎人3[ACG汉化][WiFi][日版汉化]30 - [RMHJ08]怪物猎人3[兔友汉化组][WiFi][日版汉化]31 - [RNOJ01]异色代码 R 记忆之门[ACG汉化][日版汉化]32 - [RNOJ01]异色代码 R 记忆之门[Wii吧汉化组+猫星汉化组][日版汉化]33 - [RODK01]瓦里奥制造手舞足蹈[猫星汉化][韩版汉化]34 - [RONJG9]御姐玫瑰革命[猫星汉化][WiFi][日版汉化]35 - [ROWJ08]大神[四叶草汉化][日版汉化]36 - [RQ2JK6]疯狂攀登者Wii[猫星汉化][日版汉化]37 - [RQREXJ]空中杀手无暇王牌[猫星汉化][美版日文语音]38 - [RSFJ99]胧村正妖刀传[ACG汉化][日版汉化]39 - [RSFJ99]胧村正妖刀传[贴吧中文典藏版][日版汉化]40 - [RSPW01]Wii运动[台版官中]41 - [RTNJCQ]天诛4[猫星汉化][日版汉化]42 - [RWLJ01]瓦里奥大陆摇晃[猫星汉化][日版汉化]43 - [RYAJDA]小双侠Wii 噗通噗通大赛车[猫星汉化][日版汉化]44 - [RZ9JG9]THE聚会游戏(简单2000系列2 家庭聚会)[猫星汉化][日版汉化]45 - [RZDJ01]塞尔达传说黎明公主[ACG汉化][日版汉化]46 - [RZPJ01]林克的弓箭训练[ACG汉化][日版汉化]47 - [RZTW01]Wii运动度假胜地[MP][台版官中]48 - [SB4W01]超级马里奥银河2[台版官中]49 - [SBDE08]生化危机暗黑编年史[xjsxjs197汉化][1 .0][WiFi][美版汉化]50 - [SBWJRA]育儿妈妈[猫星汉化][日版汉化]51 - [SC8J01]Wii第一次接触加强版(Wii控制器加强版动感欢乐组合)[猫星汉化][日版汉化]52 - [SL2J01]零真红之蝶[ACG汉化][日版汉化]53 - [SMNW01]新超级马里奥兄弟Wii[台版官中]54 - [SOMJ01]大家的节奏天国[猫星汉化][日版汉化]55 - [SOUJ01]塞尔达传说天空之剑[ACG汉化][日版汉化]56 - [SSQW01]马里奥派对9(马里奥聚会9)[台版官中]57 - [ST7JGD]富豪街[ACG汉化][日版汉化]58 - [STEJ18]俄罗斯方块派对豪华版[猫星汉化][日版汉化]59 - [SUKJ01]星之卡比 Wii[猫星汉化][日版汉化]60 - [SUPJ01]Wii 派对(欢乐聚会)[猫星汉化][日版汉化]61 - [SX4J01]异度之刃[ACG汉化][日版汉化]62 - wiiware [JADE]塞尔达传说:众神的三角力量[Dark_Link&kamiru2汉化][美版汉化]63 - wiiware [NAJ8]塞尔达传说时之笛[天使乐园汉化][日版汉化]64 - wiiware [WBSP]梦幻泡沫[Wiidao汉化][欧版汉化]65 - wiiware [WC2P]最终幻想水晶防线R2[Wiidao汉化][欧版汉化]66 - [DQAJK2]水瓶座棒球[猫星汉化][日版汉化]67 - [R2UJ8P]一起来拍打[猫星汉化][日版汉化]68 - [R2VJ01]罪与罚宇宙的后继者[猫星汉化][WiFi][日版汉化]69 - [R3IJ01]银河战士 PRime[TGFC汉化][日版汉化]70 - [R3OJ01]银河战士另一个M[ACG汉化][日版汉化]71 - [R4ZJ01]零月蚀之假面[ACG汉化][日版汉化]。
Real+File全目录+更新到311套

r199 Miki Kikuike 菊池 みき
r200 Ayano Oda 小田 あやの
r201 Madoka Mizuhara 水原 まどか
r202 Saki Uchida 内田 さき
r192 Yurino Ohta 大田 ゆりの
r193 Satomi Higasino 東條 さとみ
r194 Mizuki Kubo 久保 みづき
r195 Misono Kuroki 黒木 みその
r196 Tugumi Simada 島田 つぐみ
r197 Natumi Ogawa 小川 なつみ
r034 Ema Takahashi 高橋 えま
r035 Kaede Morishita 森下 かえで
r036 Nami Inaba 稲葉 なみ
r037 Eri Akiba 秋葉 えり
r038 Natsumi Kawasumi 川澄 なつみ
r039 Mizuho Suzuki 鈴木 みずほ
r139 Yuka Simada 島田 ゆか
r140 Misaki Ichijyou 一条 みさき
r141 Aoi Sawaki 澤木 あおい
r142 Mina Kawamura 川村 みな
r143 Kumi Konno 今野 くみ
r144 Aya Yamauchi 山内 あや
r145 Kanae Yasuda 安田 かなえ
r185 Youko Komatu 小松 ようこ
r186 Naho Kimura 木村 なほ
r187 Yuri Ohhara 大原 ゆり
r188 Honami Noda 野田 ほなみ
文档

せいなるゆみ 神圣弓 5 100 10 1~5 0 ○ 对飞行系和魔兽系(モンスター系)有特效
●特殊●
日文名称 中文译名 威力 命中 必杀 射程 重量 回复 备注
しょくしゅ 触手 10 90 0 1 0 魔兽系敌人专用,无法获得
ぎんのやり 银枪 8 90 0 1 1
てやり 投枪 3 70 0 1~2 2
ナイトキラー 骑士杀手 3 85 0 1 1 对骑士系(ナイト系)有特效
せいなるやり 神圣枪 3 90 10 1 0 ○ 对魔兽系(モンスター系)有特効
グラディウス 圣枪?古拉迪乌斯 15 100 0 1~2 0 ○ グラディウス=Gladius
茜莉佳装备:把以上6B03均改为6B7B即可 哈哈
日文名称 中文译名 威力 命中 必杀 射程 重量 回复 备注
つるぎ 剑 0 90 0 1 0 基本武器
はがねのつるぎ 钢剑 4 80 0 1 1
ぎんのつるぎ 银剑 8 90 0 1 1
ゆうしゃのけん 勇者之剑 5 100 30 1 0
6B03-01-10圣剑
6B03-01-11伶月剑
6B03-01-13触手
6B03-01-12王室剑
6B03-01-14钢弓
6B03-01-15圣弓
6B03-01-16银弓
6B03-01-30皮盾
6B03-01-31钢盾
6B03-01-32银盾
6B03-01-33圣盾
6B03-01-34防魔盾
6B03-01-35龙盾
6B03-01-36圣戒指
6B03-01-37天使戒指
Will社+绿茶社已汉化的游戏列表

繁体中文改版制作:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
繁体中文改版制作:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
台湾地区代理发行:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
rouge(ルージュ)
2007年01月25日 HITOZUMA JYAN 熟女麻將 繁体中文版
/web/20031127165140/www.will-japan.co.jp/rouge/gu06/gu0601.htm
PULLTOP(プルトップ)
/home.php
画师 たけやまさみ 八島タカヒロ 基井あゆむ 藤原々々
SD画师 鸟砂丘 仁之丞 田口まこと いくたたかのん
propeller(プロペラ)
/top.html
/rouge/Hito/index.htm
繁体中文改版制作:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
台湾地区代理发行:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
2007年09月25日 HITOZUMA JYAN II 熟女麻將2 繁体中文版
繁体中文改版制作:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
台湾地区代理发行:FUTURE-DIGI CO.,LTD.台灣未來數位有限公司
2010年05月28日 ONEGAI OHOSHI-SAMA 綺願幸運星 繁体中文版
/gp03/index.htm
/web/20030221224626/www.hermit-info.jp/h_top.html
/top.html
ビジネス中国语単语帐
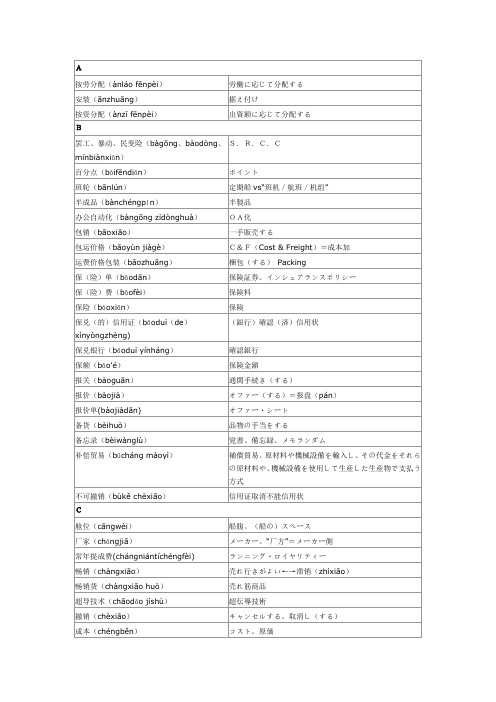
风险(fēngxiǎn)
リスク。危険
付款(fùkuǎn)
支払う。ペイメント
付款方式(fùkuǎn fāngshì)
支払方式
付款交单(fùkuǎn jiāodān)
D/P決済。支払後船積書類渡し
付款条件(fùkuǎn tiáojiàn)
支払条件
附件(fùjiàn)
付属書類。同封書類。アタッチメント
来料加工(lái liào jiāgōng)
無償材料提供をうけての委託加工
来样加工(lái yàng jiāgōng)
見本提供をうけての委託加工
劳动密集型(láodòng mìjíxíng)
労働集約型
离岸价格(lí’ànjiàgé)
FOB価格
离岸价(lí’ànjià)
FOB価格
廉价(liánjià)
為替レート
汇票(huìpiào)
為替手形
货号(huòhào)
品番
货款(huòkuǎn)
商品・貨物代金
货源(huòyuán)
商品の供給源
货源不足←→货源丰富
品不足←→商品の供給源豊富
货样(huòyàng)
商品見本。サンプル
货主(huòzhǔ)
荷主
J
基本险(jīběnxiǎn)=水渍险(shuǐzìxiǎn)
半成品(bànchéngpǐn)
半製品
办公自动化(bàngōng zìdònghuà)
OA化
包销(bāoxiāo)
一手販売する
包运价格(bāoyùn jiàgè)
C&F(Cost & Freight)=成本加
运费价格包装(bāozhuāng)
梱包(する)Packing
01-2013_-_Aawoot_Srikhaow_-_PreparationofCu2OH3NO3ZnOanovelcatalystformethylor[retrieved-2016-11-15]
Applied Catalysis B:Environmental 130–131 (2013) 84–92Contents lists available at SciVerse ScienceDirectApplied Catalysis B:Environmentalj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /a p c a tbPreparation of Cu 2(OH)3NO 3/ZnO,a novel catalyst for methyl orange oxidation under ambient conditionsAssadawoot Srikhaow a ,b ,Siwaporn Meejoo Smith b ,c ,∗aMaterials Science and Engineering Graduate Program,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand bCenter of Excellence for Innovation in Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand cDepartment of Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailanda r t i c l ei n f oArticle history:Received 4May 2012Received in revised form 1September 2012Accepted 16October 2012Available online 29 October 2012Keywords:Copper hydroxide nitrate Zinc oxideLayered hydroxyl salts Catalytic wet oxidation Wastewater treatmenta b s t r a c tThis work reports a novel process to synthesize copper hydroxyl nitrate/zinc oxide composites (Cu 2(OH)3NO 3/ZnO),and their application as a highly effective and reusable catalyst for wet oxidation of methyl orange (MO)under ambient conditions.No additional air or oxygen flow is ing a metal oxide assisted method,the Cu 2(OH)3NO 3/ZnO composites were hydrothermally obtained by varying the Cu:Zn mole ratio (2:1,4:1,and 6:1)and the structural,chemical and surface properties of the composites were investigated.Decolorization of 500ppm MO can be effectively catalyzed by the Cu 2(OH)3NO 3/ZnO composite (Cu:Zn =4:1)with the color,chemical oxygen demand (COD)and total organic carbon (TOC)removal efficiencies being greater than 99%,98%and 94%,respectively,after 20min treatments using the catalyst loading of 3g L −1.Results from systematic catalytic activity tests strongly suggested that MO was oxidized by oxygen dissolved in the dye solution,and that the degradation pathway of MO possibly occurred through radical and H 2O 2generation.The application of highly efficient Cu 2(OH)3NO 3/ZnO cat-alysts in wastewater remediation may be attractive alternative to existing oxidation catalyst systems as they are low-cost,simple to prepare,feasible to operate under ambient conditions.© 2012 Elsevier B.V. All rights reserved.1.IntroductionWater pollution is one of the main environmental concerns impacting the world ecology.Therefore,water protection plans and wastewater treatments should receive a great attention as they impact the world’s economic growth,global food production and industrial development.It is widely accepted that agricultural runoff and industrial wastewater may contain hazardous chemicals [1,2]and hence discharge of the polluted water without treatment can cause major damage to the quality of natural water reservoirs.Textile industries,in particular,intensively use chemicals (such as dyes and transfer reagents)and massive amounts of water in dyeing processes.Thus,significant amounts of dye contaminated effluent might be produced and effective treatment processes must be put in place to clean the water before releasing into waterways.One of the main research areas in wastewater treatment is developing a novel technology to effectively remove residual dyes and organic pollut-ants from wastewater [3–5].Conventional methods used to remove∗Corresponding author at:Department of Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand.Tel.:+66222015164;fax:+6623547151.E-mail addresses:siwaporn.smi@mahidol.ac.th ,siwaporn.meejoo@ (S.M.Smith).pollutants from wastewater are adsorption,biological treatment and chemical oxidation [3,6].Although adsorption is the simplest method,but less effective as pollutants were only transferred to the sorbent surface,and additional treatment of the contaminated solid is required [7].Biological treatments are considered as promis-ing alternatives.However,it may be rather difficult to biologically degrade some conjugated aromatic compounds due to their struc-tural stability [8].In addition,microorganisms may not be survived under extreme conditions (extreme pH or highly toxic)inhibiting effective biological treatments of wastewater [9,10].Advanced oxidation processes (AOPs)have been described as chemical methods operated to induce the oxidative degradation of organic compounds by radical species [11,12].Examples of AOPs include ozonation [13],Fenton process [14],photocatalysis [15],sonolysis [16]and other chemical oxidation processes induced by oxidizers such as air,O 2,O 3,and H 2O 2[11,17,18].To practically operate in industrial sites,the oxidation process should remove organic pollutants effectively under mild conditions,and with low cost.Photocatalytic oxidation and sonolysis are of interest as they can operate under ambient conditions with no requirement of additional oxidizer such as ozone and hydrogen peroxide [16,18].However,industrial integration is difficult,as these require special maintenance/operation of high power light sources,or special-ized sonication equipments.In recent years,a limited number of works have reported oxidation of organic compounds via catalytic0926-3373/$–see front matter © 2012 Elsevier B.V. All rights reserved./10.1016/j.apcatb.2012.10.018A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9285wet oxidation(CWO)at near ambient conditions,in which the organic substances undergo aerial oxidation over metal catalysts such as Fe2O3–CeO2–TiO2/␥-Al2O3[19],MoO3/Ce[20],polyoxo-molybdate nanotubes[21],and ZnO/MoO3mixed oxide nanotubes [22].Although no harmful chemical reagent is required,air or oxy-gen is necessary to activate these oxidation processes.Apart from the intrinsic properties of the catalyst,factors such as catalyst loading,concentration of the organic substrate,airflow rate(and, perhaps,its purity)can play significant roles in the efficiency and rate of the processes[5,12,23].A few copper hydroxyl salts were reported as promising cat-alysts in azo dyes removal via catalytic wet peroxide oxidation (CWPO)in which H2O2is required as oxidizer[24,25].In2010, Zhan and Chen reported the degradation of azo dyes over copper hydroxyphosphate,Cu2(OH)PO4,under near-neutral pH conditions [24],whereas copper hydroxide nitrate,Cu2(OH)3NO3is an effec-tive CWPO catalyst for oxidative degradation of azo dyes in a wide pH range[25].Previously reported,Cu2(OH)3NO3can be synthe-sized by several routes,such as precipitation of Cu(NO3)2in a basic aqueous solution[26],urea hydrolysis of Cu(NO3)2[27],chemical reaction between CuO and aqueous Cu(NO3)2solution[28],cation exchange of Mg(II)for Cu(II)in aqueous Cu(NO3)2solution and evaporation of aqueous Cu(NO3)2solution[29].To gain a significant advantage over the previously reported cop-per hydroxyl salt catalysts,an ideal catalyst should enable organic substrates to be oxidized under ambient conditions,with no requirement of additional continuously supplied oxidizing agent. In this work,Cu2(OH)3NO3(a copper hydroxyl salt)was incor-porated with microcrystalline ZnO by a one-step hydrothermally metal oxide assisted method.This study also reports the application of this material(denoted as Cu2(OH)3NO3/ZnO)as a highly effec-tive and reusable catalyst for wet oxidation of methyl orange(MO) under ambient conditions without addition of oxidizer.Details on systematic investigations of the structural and catalytic activity of this material and possible degradation pathway of MO are dis-cussed.2.Experimental2.1.MaterialsAll chemicals used in this work were commercially supplied as analytical grade reagents,and used without further purification. De-ionized water was used throughout the experiments.Copper nitrate trihydrate and copper sulphate pentahydrate were com-mercially obtained from Univar,while ZnO powdered material was obtain from Merck.Tert-butanol(Merck)and NaOH(Rankem)were employed as hydroxyl radical scavenger reagents.2.2.Preparation method offlower-like ZnOA d-glucose assisted precipitation method[30]was applied to obtainflower-like ZnO materials by using NaOH and Zn(NO3)2 (Qrëc)as starting reagents,a mixture of H2O:acetone:ethyl acetate (3:3:2by volume)as co-solvent,and d-glucose(Univar)as ion sta-bilizer.Obtained white precipitate was subsequently calcined at 400◦C for3h in an air atmosphere,giving aflower-like ZnO sample with S BET=11.7m2/g.2.3.Preparation of the Cu2(OH)3NO3/ZnO samplesA series of suspensions containing theflower-like ZnO(or com-mercial ZnO)powder in Cu(NO3)2aqueous solutions were obtained by varying the Cu:Zn molar ratios(2:1,4:1and6:1).Next,these sus-pensions were sonicated for30min in an ultrasonic bath,followed by a hydrothermal treatment at100◦C in a50mL Teflon-lined stain-less steel autoclave for30min.Subsequently,the reaction mixtures were left to stand at room temperature,allowing the suspensions to cool down.Afterfiltering and washing with de-ionized water, the precipitates were freeze dried,and kept in a dry condition at room temperature.2.4.Sample characterizationStructural properties of as-prepared samples were stud-ied on a Bruker(AXS model D8advance)powder X-ray diffractometer equipped with Cu K␣radiation, =1.5419˚A,2Ârange=5–50◦,step=0.050◦,scan step=1s/step.FT-IR spectra were obtained on a Perkin Elmer(Spectrum GX FT-IR System) fourier-transform infrared spectrometer.The microstructure of catalyst samples was examined on a JEOL(JSM-6400)scan-ning electron microscope.Furthermore,energy dispersive X-ray (EDX)microanalyses were carried out to identify the chemi-cal composition of catalyst samples.The surface properties were studied by using Brunauer–Emmett–Teller method(BET Model Quantachrome/Autosorb-1,Thermo Finnigan/Sorp-tomatic1990). The concentration of elements in the samples was also determined on an X-rayfluorescence spectrometer(Bruker S4EXPLORER) equipped operating in a He working mode(X-ray tube win-dow=75m;excitation=4kW)using a loose powder preparation method and a34mm sample cup.2.5.Catalytic degradation experimentsAll catalytic reactions were carried out using the same catalyst loading;3g L−1of aqueous methyl orange(MO,500ppm)under stirring.The color removal efficiency of MO was monitored as a function of time by measuring absorbance of the dye solution after catalytic treatment at given time intervals.In order to terminate the reaction at specific reaction times,the catalyst was immediatelyfil-tered off using a Buchner funnel equipped with a water aspirator pump.UV–vis absorption spectra of the dyefiltrates were recorded on a Perkin Elmer(Lamda800)UV–vis spectrophotometer.Subse-quently,the concentration of MO in thefiltrates was quantified using the absorbance at464nm(corresponding to unreacted MO) and a curvefitting method using the Beer Lambert law.The color removal efficiency(Á,%)of methyl orange was calculated by using this equation:Á(%)=C0−C tC0×100(1)where C0is the initial concentration of MO and C t is the concentra-tion of MO after‘t’min.Total organic carbon(TOC)content in the dye solutions was determined by an in-house method(SGS laboratory service):LBEN-09149based on United States Environmental Protection Agency, 2004,EPT method9060A.Moreover,chemical oxygen demand (COD)was measured by a standard closed reflux/titration method [31].The TOC removal efficiency is defined as:TOC removal efficiency(%)=TOC0−TOC tTOC0×100(2)where TOC0is initial TOC of the solution and TOC t is TOC of solution after‘t’min reaction time.Similarly,COD0and COD t values were used instead of TOC0and TOC t values in Eq.(2)respectively,to calculate COD removal efficiencies.The data from triplicate mea-surements were analyzed to obtain average values and standard deviation(SD).The significance of difference of data was evaluated using the Student’s T-test and one-way ANOVA[32]at a significance level of0.05.86 A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–92To investigate the possible mechanism of decolorization of MO, the experiments were conducted under air atmosphere,vacuum in a closed system and in the presence of radical scavengers i.e.tert-butanol[33,34]and NaOH[35],into the solution at25◦C.Detailed processes are described in the supplementary data.The stability of catalyst,Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)was studied by monitoring the generation of metal ions in the dye solution during catalytic wet oxidation.After20-min reaction,the concentration of Cu and Zn ions in the dyefiltrates was then deter-mined by using a graphite furnace atomic absorption spectrometer (GFAAS,Perkin Elmer AAnalyst100).A hollow cathode zinc lamp (Perkin Elmer)operated with10-mA current was employed,with argonflow throughout the heating program,except during the atomization step.2.6.Characterization of the degradation productsLiquid chromatography with ion trap mass analyzer(LC–MS, Agilent technology,Agilent1100equipped with Esquire3000plus) was employed to detect the degradation products upon the oxi-dation of methyl orange.The LC–MS system was equipped with C18column and30%of acetonitrile and70%of0.01M ammonium acetate(pH6.8)were used as a mobile phase.Theflow rate used was0.6mL min−1.The mass spectrometer was equipped with an electrospray ionization(ESI)source operating at negative polar-ity.This LC/MS system could detect mass ranged between100and 400m/z.3.Results and discussion3.1.Characterization of the catalystsPowder X-ray diffractrograms of ZnO powder and the syn-thesized Cu2(OH)3NO3/ZnO samples with varying Cu:Zn mole ratios are shown in Fig.1a.The diffraction peak at∼13◦corre-sponds to the basal distance(6.96˚A)typically reported for the Cu2(OH)3NO3layered materials[26,27,29,36].It was observed that the Cu2(OH)3NO3/ZnO derived from the Cu:Zn molar ratio=2:1 contains two crystalline phases,monoclinic Cu2(OH)3NO3(JCPDF card no.74-1749)and hexagonal ZnO(JCPDF card no.36145).How-ever,with the increased Cu:Zn molar ratios to4:1and6:1,the structural characteristics of Cu2(OH)3NO3becomes more evident, whereas the diffraction peaks corresponding to the ZnO phase(at 34.4◦and47.4◦)become weaker in intensity.This is possibly due to a full coverage of Cu2(OH)3NO3layers on the ZnO particles.The sharp and well-defined peaks reflected high degree of crystallinity for all synthesized samples.No diffraction peaks corresponding to neither Zn(OH)2,CuO nor Cu(OH)2phases were observed.It should be pointed out that,to our knowledge,this metal oxide assisted route to synthesize copper hydroxide nitrate has never been reported.The conversion of copper nitrate to copper hydroxyl nitrate possibly resulted from the availability of hydroxyl groups on the ZnO solid base.In a previous study,the basic strength of a ZnO sample was reported as7.2<H<9.3in an Hammett indicator scale, indicating a fairly high strength comparing with those of ZrO2,TiO2, CaO and SrO[37].From Fig.1b,IR measurements also confirm the formation of copper hydroxide nitrate in the system studied.In a good agree-ment with previously reported works[26–29]the IR peaks at876, 785and676cm−1can be assigned to hydrogen bonding frequencies related to Cu O H.The peaks at1048( 1),810( 2),1340,1348and 1429cm−1( 3)can be attributed to the vibration modes of NO3−ions[27].The symmetric and asymmetric stretching modes of NO3−at1429and1340cm−1suggested the presence of NO3−between copper hydroxide layers.The IR band at1048cm−1corresponds to the N O stretching vibration of a monodentrate O NO groups[38],Fig. 1.(a)Powder XRD patterns of Cu2(OH)3NO3/ZnO samples at varying the Cu:Zn molar ratios in comparison to that of ZnO and(b)Infrared spectra of Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)and ZnO powder.whereas the band at1637cm−1can be ascribed to a HOH bending mode.The peaks at3543cm−1and3433cm−1indicated more than one type of hydroxyl groups in the structure[27,29].Note that the characteristic peak of ZnO at430wavenumber[39]was not clearly observed in the Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)sample,possibly due to signal overlapping and the full coverage of Cu2(OH)3NO3 layers formed on the ZnO particles as previously discussed.Noticed from Fig.2a,the microcrystalline ZnO substrate resem-bles to a bunch of doubleflowers.Although the Cu2(OH)3NO3/ZnO composites did not retain theflower-like microstructure of their substrate(Fig.2b–d)a resemblance of aggregates offlake-like plates(∼400nm in thickness)morphology can be still observed. X-rayfluorescence(XRF)was employed to perform elemental anal-ysis in the composite samples,and the results were included in Table1.The XRF results suggest that the content of Cu was found to increase in the composites with increased Cu:Zn molar ratios.This finding was consistent with the results from EDX microanalyses, Fig.3,revealing the presence of Cu and Zn on the composite surface, and that the content of Cu was found to be higher in the compositesA.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9287Fig.2.SEM images of theflower-like ZnO(a)and(b–d)Cu2(OH)3NO3/ZnO at the Cu:Zn molar ratios of2:1,4:1and6:1,respectively.Table1Elemental concentration in the Cu2(OH)3NO3/ZnO samples at various Cu:Zn molar ratios obtained by XRF analysis.Cu:Zn Concentration(wt%)Depth ofpenetration(m) Cu Zn2:147.97±0.04815.09±0.0140.13–0.164:149.86±0.04811.30±0.0120.14–0.176:160.09±0.054 1.59±0.0040.13–0.16 derived from the higher Cu:Zn.However,as shown in Table2,the Cu:Zn ratios vary in different areas of the sample surface,suggest-ing that the Cu-containing compound does not homogeneously incorporated with the ZnO particles.3.2.Catalytic activity of Cu2(OH)3NO3/ZnOThe performance in decolorization of500ppm aqueous methyl orange(MO)solution over the Cu2(OH)3NO3/ZnO composites at varying Cu:Zn ratios were examined over a period of time as shown in Fig.4a.Notably,the color removal efficiencies reached99% within1.5min of the treatments by all composites at25◦C under atmospheric pressure(Fig.4a).It was noticed from Fig.4a and b,that the Cu2(OH)3NO3/ZnO composites prepared with relatively high Cu:Zn molar ratios oxidized MO slightly faster than the sam-ples having lower Cu content,implying that the amount of copper may play a crucial part to the reaction kinetics of MO degradation. Determined usingfirst-order kinetic model,it was found that the higher rate constants for the catalytic wet oxidation of MO were obtained from the Cu2(OH)3NO3/ZnO with the higher Cu:Zn molar ratios(6:1,4.4min−1;4:1,3.9min−1;2:1,3.6min−1).Thefirst order kinetic plot in Fig.4b was focused in the range of shorter reaction time,because after1.5min the color removal efficiencies reached 99%.Statistical analyses suggested that the kinetic constants for the MO decoloriaztion by each catalyst(2:1,4:1and6:1)are signifi-cantly different due to the Cu:Zn molar ratios at the level of p<0.05. Fig.4c shows a characteristic absorption band at464nm corre-sponding to a conjugated azo bond structure in the MO molecule [40].In this work,the absorption band at464nm become weaker in intensity after treatment with Cu2(OH)3NO3/ZnO composites. Therefore,in consistent with the result in Fig.4b,the UV–vis spec-tra of MO after1-min treatments over the composites at varying ratios indicate that the composites with higher Cu:Zn molar ratios lead to the higher color removal rates.Chemical oxygen demand(COD)and total organic carbon(TOC) values are generally determined to examine the water quality. According to the results(Fig.5)COD removal efficiencies over theTable2EDX analysis in different areas of the Cu2(OH)3NO3/ZnO samples at various Cu:Zn molar ratios.Cu:Zn%ElementArea#1Area#2Area#3Cu Zn O Cu Zn O Cu Zn O2:125.7414.8059.4639.85 5.5254.6323.5226.3050.18 4:137.78 5.1057.1145.08 1.9053.0154.09 2.8243.08 6:153.70 3.4042.9048.12 1.4850.4035.73 1.3262.9588 A.Srikhaow,S.M.Smith /Applied Catalysis B:Environmental 130–131 (2013) 84–92Fig.3.EDX analysis of the Cu 2(OH)3NO 3/ZnO samples at various Cu:Zn molar ratios (a)2:1,(b)4:1and (c)6:1,respectively.catalyst are about 88%and 98%after treatment for 5and 20min,respectively.In addition,the results in Fig.5also suggest organic carbon mineralization and CO 2evolution from the oxidation of MO after 5-and 20-min of the treatments by Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)resulting in 84%,and 94%TOC removal efficiencies,respectively.Thus,at this catalyst loading condition,the effective decolorization of MO occurred through the fragmentation of the dye into some other colorless compounds,as well as,the mineral-ization of MO.3.3.Possible mechanism and degradation pathwayThe BET surface area of catalysts prepared by the Cu:Zn molar ratios of 2:1,4:1,6:1are 10.81,8.85,and 5.76m 2/g,respectively.From Fig.4a,the Cu 2(OH)3NO 3/ZnO with lower specific surface area gave the higher color removal efficiency,suggesting that the effective color removal was not due to adsorption of dye onto the solid surface.It was found that the higher performance catalysts have lower surface areas,and thus the MO degradation rates are not proportional to the BET surface area of the catalyst.This maybe because copper hydroxyl nitrate deposited on the surface and filled in the pores of the ZnO,resulting in the materials with lower surface areas.It should be also pointed out that the lower surface area materials also have reduced pore volumes,as the pore volumes of catalysts prepared by the Cu:ZnmolarFig.4.(a)Color removal efficiency upon time using Cu 2(OH)3NO 3/ZnO catalysts as a function of Cu:Zn molar ratio and surface area (m 2/g),(b)kinetic of methyl orange oxidation catalyzed by Cu 2(OH)3NO 3/ZnO as a function of Cu dosage and (c)UV–vis absorption spectrum of fresh MO (50ppm)and those of oxidized MO after 1-min treatment by the catalysts with various Cu:Zn molar ratiosunder ambient conditions.Initial concentration of MO =500ppm;catalyst loading =3g L −1.ratios of 2:1,4:1,6:1are 0.08,0.07,and 0.02cc/g,respectively.In addition,approximately the same color removal efficiency (∼99%)was also observed when a dispersion of Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in aqueous MO solution was kept in the dark under similar experimental conditions mentioned above.Thus,light had no influence to the catalytic activity of composite.As a result,one could suggest a catalytic wet oxidation (CWO)process,in which the dye undergoes aerial oxidation over the composites.Note that,commercially supplied ZnO powder can also be replaced the flower-like ZnO to produce the Cu 2(OH)3NO 3/ZnO composites,A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9289Fig. 5.Color(Á),COD and TOC removal efficiencies upon treatment of MO aqueous solution by the Cu2(OH)3NO3/ZnO(Cu:Zn=4:1).Initial concentration of MO=500ppm;catalyst loading=3g L−1.having almost the same catalytic activity.In an attempt to under-stand the nature of MO degradation over the Cu2(OH)3NO3/ZnO composites,additional MO degradation reactions were conducted in various experimental conditions and reported in Fig.6.It is well known that CWO catalysts require oxygen to degrade organic compounds.Accordingly,if catalytic wet oxidation(CWO) was the major process responsible for MO degradation,the oxi-dation rate of MO should be directly proportional to the oxygen concentration.One of the experiments was conducted under vac-uum(setup Fig.S2)here the MO solution was thoroughly degassed prior to being used.As reported in Fig.6,the color removal effi-ciency of MO under vacuum was about7.72%,which is much lower than under ambient conditions(∼100%).The proposed mechanism of CWO reactions outlined by Ma et al.[44]may be applied to describe the CWO reactions occurring here.RH+Cu2+→R•+Cu++H+(3) Cu++O2→Cu2++O2−(4) 2O2−+2H2O→2OH−+H2O2+O2(5) H2O2+Cu2+→HO•+OH−+Cu2+(6)HO•+MO→degradationproducts(7)parative results of the color removal efficiency after5min treatment of MO with Cu2(OH)3NO3/ZnO(Cu:Zn=4:1),with varying experimentalconditions.Fig.7.Powder XRD patterns of Cu2(OH)3NO3/ZnO samples(Cu:Zn=4:1)before and after reaction comparing with that of MO.From the proposed model,Cu(II)in the catalyst undergoes reduction reaction forming Cu(I)which further reacts with oxygen dissolved in an aqueous solution.Subsequently,H2O2is generated as intermediates through the reaction of O2−and water molecules. Following this model,it is possible that hydroxyl radical is cre-ated when Cu2(OH)3NO3decomposed H2O2intermediates.Finally, MO molecule was attacked by hydroxyl radicals.According to the proposed CWO reaction mechanism,the presence of radical scavenging reagents such as NaOH and tert-butanol,should signif-icantly inhibit the oxidation of MO.The result in Fig.6represents that adding NaOH and tert-butanol gave low color removal effi-ciencies of2.40%and5.95%,respectively after5-min treatments using Cu2(OH)3NO3/ZnO(Cu:Zn=4:1).These results strongly sup-port that the decomposition of MO occurred through a radical pathway.Previously discussed from the result in Fig.4c,the decrease in intensity of the absorption band corresponding to MO indi-cated the cleavage of the azo group,and hence decolorization of the dye solution.No spectral shift corresponding to possible complexation between dye molecules and metal cations[41–43] was observed in our system.Besides this,Fig.7shows that the Cu2(OH)3NO3/ZnO catalyst undergoes no significant structural change after20min reaction.Apart from the typical features cor-responding to the Cu2(OH)3NO3crystalline phase,extra diffraction peaks were observed at8.9◦and17.1◦which correspond to crystal-lized MO on the catalyst surface.The observed slight shift in peak positions is possibly due to microstrains on the sample occurring during the drying process.According to XRD results,there is no evidence of any new crystalline phase in the used catalyst,ruling90 A.Srikhaow,S.M.Smith /Applied Catalysis B:Environmental 130–131 (2013) 84–92Fig.8.Possible MO degradation pathway producing molecular fragments as detected by LC/MS.out the possibility of complexation between the Cu 2(OH)3NO 3/ZnO composite and MO.Consequently,all results discussed above sup-port the degradation of MO over Cu 2(OH)3NO 3/ZnO composites via a catalytic wet oxidation process.Nevertheless,in contrast to conventional CWO catalysts,Cu 2(OH)3NO 3/ZnO is highly active with no requirement of air or oxygen flow or any additional oxidant.In an attempt to determine the nature of MO degradation prod-ucts,LC/MS analysis of decolorized MO solutions revealed the presence of three chemical species after a 5min reaction period.At level of 99%decolorization,the chemical species identified were unreacted MO (M w =304)and two product species with m /z =290and 208,corresponding to MO fragments (Fig.S3).The reaction steps in wet oxidation of MO catalyzed by Cu 2(OH)3NO 3/ZnO observed by LC/MS are given in Fig.8.3.4.Catalyst reusabilityThis part focuses on possibility of recovery,recyclization,and regeneration of the catalyst.By simple centrifugation and decanta-tion,it was found that the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)catalyst can be reused for three consecutive runs,without any further treat-ment,maintaining the color removal efficiencies of 99%,and the COD and TOC removal efficiencies of greater than 90%after 20-min reaction as shown in Fig.9a.As previously discussed,adsorption of MO on the catalyst surface occurred.However,based on the high color removal efficiencies for three cycle utilization,the presence of adsorbed MO on the surface of used catalyst did not affect the color removal efficiencies.Nevertheless,when the catalyst was fur-ther reused without treatment in the 4th run,it was found that the color removal efficiency dropped from 99%to about 37%as reported in Fig.9b.Therefore,the used catalyst after the 3rd run requires suitable regeneration prior to further use.Possible regen-eration methods include refluxing method,calcination under a suitable atmosphere,rinsing by appropriate solvent or some combi-nations of processes [12,19,45,46].Due to the low thermalstabilityFig.9.(a)Color (Á),COD and TOC removal efficiency for suspension of the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in MO aqueous solution during consecutive runs and (b)comparative results of the color removal efficiencies (Á)of MO by the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in the 1st and 4th cycles without regeneration,and that of the 4th cycle obtained by employing the regenerated catalyst after the 3rd run via mild acid wash.Initial concentration of MO =500ppm;catalyst loading =3g L −1.of copper hydroxide nitrate and the solubility of metal oxide in acid,the spent Cu 2(OH)3NO 3/ZnO was regenerated by washing with weak acid to remove the unreacted MO and,possibly,degra-dation products adsorbed on the catalyst surface without causing serious damages to the catalyst.It was found that,after filtration,washing the spent catalyst (from the 3rd run)with 5mM HCl(aq)for 20min,and rinsing with water followed by drying at 100◦C,the regenerated catalyst can be employed in the 4th cycle giving the color removal efficiency of 95%as shown in Fig.9b.This color removal efficiency is lower than those obtained from the first three cycles,probably due to some loss of active copper species during acid washing.Table 3reports that,after 20-min MO degradation,the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)catalyst slightly dissolved in the dye solution giving the concentration of Cu and Zn ions of 4.3and 0.5ppm in the first cycle.In the subsequent cycles,the solubil-ity of catalyst was found lower.According to the high color,COD,and TOC removal efficiencies (>90%)for three cycle utilization,this trace amount of meal leaching did not affect the removal efficien-cies,implying that the Cu 2(OH)3NO 3/ZnO catalyst was stable for three consecutive cycles with no requirement of catalyst regen-eration (Fig.9a).However,due to the results of metal leaching,there may be questioning of the MO degradation via homogeneous。
hymmnos语 中文字典
ヒュムノス単語意味発音流派Tanta 舞蹈タンタ正纯(共)Yuez 相互ユェゼ正纯(共)Zauve 一个瑞利ザーベ古月咒(古代语)0 0 ネル/オ(二進法)正纯(共)1 1 ノイ/イ(二進法)正纯(共)10 10 デク/デ正纯(共)100 100 ヘク/ヘー正纯(共)1000 1000 キク/キィ正纯(共)10000 10000 ミク/ミィ正纯(共)2 2 ジ正纯(共)3 3 ドリ正纯(共)4 4 フェフ正纯(共)5 5 ヴィラ正纯(共)6 6 イクサ正纯(共)7 7 ヘプト正纯(共)8 8 オクタ正纯(共)9 9 ネイ正纯(共)<-x 这 (ス)パグ新约(Pasutaria律)ChronicleKey 编年史键 Hymmnos a律(EOLIA属)Dia 王、王座、玉座、支配者ディア正纯(共)Fou 稍微(第Ⅰ想音)フォウ正纯(共)Harmonius 进行合音ヒュムノス正纯(共)Linca 链接程序ヒュムノス正纯(共)Ma 平常心(第Ⅰ想音)マ正纯(共)Manac 真名、灵魂的名字、御魂名マナク古月咒(古代语)Metafalica メタファリカ metafalis律(神圣语)Nn 无気力状态(第Ⅰ想音)ン正纯(共通语)None None None 正纯(共通语)Paja 清洗剂パージャ正纯(共)Phantasmagoria 千变万化的风ファンタスマゴリアa律(EOLIA属)Re=Nation 国家リネイション正纯(共)Rig=Veda 吠陀リグヴェーダ metafalis律(神圣语)Rrha 恍惚状态(第Ⅰ想音) [ルル]ラ正纯(共)Suspend 暂停サスペンド正纯(共)Was 很、非常强烈的(第Ⅰ想音)ワス正纯(共)Wee 颇、相当的(第Ⅰ想音)ウィ正纯(共)a.u.k. 相当于动词アウク新约(Pasutaria律)accrroad 带来えるアクロード正纯(共)acra 当然、肯定アクラ正纯(共)adyya 今日アディーア正纯(共)afezeria 祝福するアフェゼリア正纯(共)ag 而且、和アグ新约(Pasutaria律)aiph 如果アィフ正纯(共)aje 新アジェ新约(Pasutaria律)akata 故事アカタ正纯(共)ale 声音アーレ新约(Pasutaria律)alroetsue 赎回、赎罪アゥロエットューエ metafalis律(神圣语)ammue 声波(声)波動アミュ古月咒(古代语)an ~再加上アン正纯(共)anw ~をアンワ、アンゥ metafalis律(神圣语)apea 沉浸于幸福中(第Ⅱ想音)アペア古月咒(古代语)ar 只有一个アル正纯(共)arhou 希望アーフー新约(Pasutaria律)arrya 箭头アリャ正纯(共)arsye 共享アーシェ正纯(共)art 通过アルト正纯(共)au伤心 a律(EOLIA属)aulla 开放、公开、泄露アウラ正纯(共)ayulsa 永远アユルサ新约(Pasutaria律)balduo 黑暗的、看不见的バルドゥー新约(Pasutaria律)bale 球バレ正纯(共)bansh (粗暴的)打开(门)バンシュ正纯(共)basilic 大砲バジリク正纯(共)bautifal 美丽的バティファル正纯(共)beja 脏ベジャ古月咒(古代语)beng 以前(時間的)前ベング正纯(共)bengnuih黄昏ベヌイ正纯(共)bexm (時間)来访问ベズム正纯(共)biron 遵循ビロン正纯(共)bister 野兽ビステル正纯(共)boches 口ボチェス正纯(共)boh 爆炸、超自然ボッフ metafalis律(神圣语)brinch 枝ブリンク正纯(共)briyante 欢乐之声ブリイャンテ metafalis律(神圣语)burle 蓝色ブゥレ正纯(共)c.z. 改变スズ新约(Pasutaria律)cause 诅咒、折磨カウゼ metafalis律(神圣语)cecet 屏蔽ケケト正纯(共)ceku 朋友、同胞セク新约(Pasutaria律)celetille 明亮、清新、美丽的セレティル正纯(共)celle 高天セルレ正纯(共)cenjue 改变チェンジュエ metafalis律(神圣语)cerchio 潜力チェルキオ正纯(共)cest 真实的、真正的、真理セスト正纯(共)cexm (人或物体)前来参观セズム正纯(共)chanti 赞誉、好评シャンティ正纯(共)chiess 吻チェス正纯(共)chs 来~ チス正纯(共)chsee 变化~るチーセ正纯(共)chyet特别,我被选中シェッ古月咒(古代语)cia 天空シア新约(Pasutaria律)ciel 天空(意译)世界シェール正纯(共)ciellenne 天空シエルレーニェ正纯(共)clalliss 给颜色、颜色クラリス正纯(共)clamour 膝盖クラマー正纯(共)clare 透明清楚クラレ正纯(共)clemezen 发疯、发狂的クレムゼン正纯(共)clyncye 纯粹、无色クリンシェ古月咒(古代语)colga 冰クオルガ正纯(共)crannidale 共享クラニダーレ metafalis律(神圣语)crudea 痛苦、苦恼クルーデア metafalis律(神圣语)crushue 纺クルシューエ metafalis律(神圣语)cupla 罪キュプル正纯(共)cyuie 痛苦、可悲的キュイ正纯(共)cyurio 管理、秩序キュリオ正纯(共)d.n. 舞蹈ドゥン新约(Pasutaria律)d.z. 死亡ズ新约(Pasutaria律)daedu 丑陋的ダェドゥス新约(Pasutaria律)dand 门ダンド正纯(共)dauan 黎明ダウアン正纯(共)dauane 曙光ダウアネ正纯(共)deata 判决、裁决、断罪ダーテ古月咒(古代语)deggeez 背叛、不服从ディジーズ正纯(共)degle 歼灭デグル古月咒(古代语)dejuy 赎罪デジュィ新约(Pasutaria律)deleir 灾祸デレイア a律(EOLIA属)delij 不愿意的、恨 a律(EOLIA属)den し但是デン正纯(共)denera 坏事デネラ正纯(共)der通过在~ デァ metafalis律(神圣语)desfel 恨、不愿意ィスフェル metafalis律(神圣语)dhezeall 俘虏、被囚禁的人ディージアル metafalis律(神圣diasee 神的儿子ディアズィエー metafalis律(神圣语)didalia 咒语ダイダリア古月咒(古代语)dilete 神的加持ディレーテ正纯(共)discest 伪装ディセスト正纯(共)dius 宝贵的、虔诚的ディウス古月咒(古代语)diviega 圣灵的宝剑、大智慧ディヴィエーガ古月咒(古代语)dn 在~ ドゥン新约(Pasutaria律)doodu 大地ドードゥ新约(Pasutaria律)dople 消除ドプレ正纯(共)dor 大地、大陸ドール正纯(共)dorn 树ドルン正纯(共)dornpica 坚果ドゥルンピカ古月咒(古代语)drone下载、捕获ドゥローネ正纯(共)dsier 欲望ヂェア正纯(共)du 一~ ドゥ新约(Pasutaria律)dyya 日(英語のDay)ディーア正纯(共)dyyal 日~ ディーアル正纯(共)eazas 彼此イーザス正纯(共)echrra 共鸣エクラ正纯(共)ee 伟大的、荣誉的ィエエ metafalis律(神圣语)eetor彼岸エトゥー正纯(共)eje 心灵、情感エジェ新约(Pasutaria律)elle 从~ エル正纯(共)elye 尽管如此、仍然エリェ新约(Pasutaria律)en 而且、因为~ エン正纯(共)enclone 结束语エンクロゥネ metafalis律(神圣语)endia 停止。
秘密岚目录 ひみつの岚ちゃん!
[124]110317 ひみつの嵐ちゃん!(未公开特辑 VIP ROOM ランキングダービー)[123]110310 ひみつの嵐ちゃん!(VIP ROOM 栗山千明/排行赛马)[122]110303 ひみつの嵐ちゃん!(VIP ROOM Aiko/人体模特~勝負Jeans~Guest:品川ヒロシ、佐藤隆太)[121]110224 ひみつの嵐ちゃん!(VIP ROOM 长谷川润/スーパーマリオネット嵐最高のキス)[120]110217 ひみつの嵐ちゃん!(VIP ROOM 桐谷美玲/人体模特~情侣搭配~Guest: アンジャッシュ(児島一哉、渡部健))[119]110210 ひみつの嵐ちゃん!(VIP ROOM 藤原紀香/排行赛马)[118]110203 ひみつの嵐ちゃん!(VIP ROOM 菅野美穗/イッパツギャンブラー岚松山健一)[117]110127 ひみつの嵐ちゃん!(VIP ROOM 吉高由里子/人体模特~连帽衫复仇~Guest: 藤本敏史、庄司智春)[116]110120 ひみつの嵐ちゃん!(VIP ROOM 仲間由紀恵/紧急企划:樱井翔的时尚修行)[115]110113 ひみつの嵐ちゃん!(VIP ROOM 宮里藍/排行赛马)★=====2010年=====★[114]101223 ひみつの嵐ちゃん!(VIP ROOM 芦田愛菜/人体模特~加入红色的成熟圣诞派对服装~)[113]101216 ひみつの嵐ちゃん!(超级提线木偶/人气嵐!差劲嵐!)[112]101209 ひみつの嵐ちゃん!(VIP ROOM 山田優/人体模特~冬季外出服搭配~Guest:徳井義実、綾部祐二)[111]101202 ひみつの嵐ちゃん!(VIP ROOM 倖田來未/一发决胜负嵐)[110]101125 ひみつの嵐ちゃん!(VIP ROOM 倉木麻衣/大人のぬり絵)[109]101118 ひみつの嵐ちゃん!(VIP ROOM 小雪/排行赛马)[108]101111 ひみつの嵐ちゃん!(VIP ROOM 戸田恵梨香/人体模特~最强约会服装~Guest:小栗旬、笠原秀幸)[107]101028 ひみつの嵐ちゃん!(人体模特对抗赛~秋季联谊时的决胜服装~Guest:关8)[106]101021 ひみつの嵐ちゃん!(VIP ROOM1 绫濑遥/VIP ROOM2 苍井优)[105]100916 ひみつの嵐ちゃん!(VIP ROOM 深田恭子/一发决胜负嵐)[104]100909 ひみつの嵐ちゃん!(VIP ROOM 吉瀬美智子/Doubt Action)[103]100902 ひみつの嵐ちゃん!(VIP ROOM 土屋ANNA/人体模特~秋季小进攻服装~Guest:DAIGO、劇団ひとり)[102]100826 ひみつの嵐ちゃん!(人体模特特别篇~夏季决胜约会服装! in 越谷AEON LakeTown~)[101]100819 ひみつの嵐ちゃん!(VIP ROOM 新垣結衣/排行赛马)[100]100812 ひみつの嵐ちゃん!(VIP ROOM 未公开TALK!SP)[099]100805 ひみつの嵐ちゃん!(VIP ROOM 金榮兒/新企划!一发决胜负嵐)[098]100729 ひみつの嵐ちゃん!(VIP ROOM 多部未華子/人体模特~海边约会服装~Guest: オードリー)[097]100722 ひみつの嵐ちゃん!(VIP ROOM Perfume)[096]100715 ひみつの嵐ちゃん!(VIP ROOM 中島美嘉/人气嵐!差劲嵐!Guest:東山紀之)[095]100708 ひみつの嵐ちゃん!(VIP ROOM 千原ジュニア/人体模特对抗赛~夏季联谊时的决胜服装~Guest:自恋刑警Team/排行赛马)[094]100701 ひみつの嵐ちゃん!(VIP ROOM 比嘉愛未/人体模特对抗赛~夏季联谊时的决胜服装~Guest: はんにゃ、ロッチ、狩野英孝)[093]100617 ひみつの嵐ちゃん!(VIP ROOM 西野カナ/人体模特~融入花纹元素的搭配~Guest:JOY、梅しゃん)[092]100610 ひみつの嵐ちゃん!(VIP ROOM 仲里依紗/Doubt Action)[091]100527 ひみつの嵐ちゃん!(VIP ROOM 黒木メイサ/排行赛马)[090]100520 ひみつの嵐ちゃん!(VIP ROOM 香椎由宇/人体模特~夜游约会~Guest: 武田修宏、パンツェッタ・ジローラモ)[089]100513 ひみつの嵐ちゃん!(VIP ROOM 森泉/Doubt Action)[088]100506 ひみつの嵐ちゃん!(VIP ROOM 佐々木希/人体模特~春季联谊时的决胜服装~Guest: キャイ~ン(天野ひろゆき、ウド鈴木)、藤本敏史、庄司智春、藤森慎吾)[087]100429 ひみつの嵐ちゃん!(人体模特特别篇~决胜的约会服装! in 御殿場~)[086]100415 ひみつの嵐ちゃん!(VIP ROOM1 島田紳助/人气嵐!差劲嵐!/VIP ROOM2 阿部寛)[085]100318 ひみつの嵐ちゃん!(VIP ROOM SP 未公开Talk/排行赛马)[084]100311 ひみつの嵐ちゃん!(VIP ROOM 北川景子/人体模特~融入动物元素的搭配~Guest:ゴリ)[083]100304 ひみつの嵐ちゃん!(VIP ROOM 中川翔子/Doubt Action)[082]100225 ひみつの嵐ちゃん!(VIP ROOM 北乃きい/人体模特~冬季时去娱乐场所的服装~Guest:DAIGO、JOY)[081]100218 ひみつの嵐ちゃん!(VIP ROOM 香里奈/烦恼顾问Guest:石田純一)[080]100211 ひみつの嵐ちゃん!(VIP ROOM 柴咲コウ/人体模特~情人节时希望男友穿的服装~Guest: 徳井義実、福田充徳)[079]100204 ひみつの嵐ちゃん!(VIP ROOM 木村カエラ/Doubt Action)[078]100128 ひみつの嵐ちゃん!(VIP ROOM1 梨花/VIP ROOM2 杏)[077]100114 ひみつの嵐ちゃん!(VIP ROOM 横峯さくら/人体模特~2010年初次约会时想穿的服装~Guest:ふかわりょう、山里亮太审查员:ドラマ「特上カバチ」堀北真希、前田美波里、小原正子、田丸麻紀、森脇英理子)★=====2009年=====★[076]091217 ひみつの嵐ちゃん!(VIP ROOM 堀北真希/人体模特~圣诞节约会时希望男友穿服装~Guest: 陣内智則、狩野英孝)[075]091210 ひみつの嵐ちゃん!(VIP ROOM 相武紗季/Doubt Action)[074]091203 ひみつの嵐ちゃん!(VIP ROOM 吹石一恵/人体模特~冬季外出时的搭配~Guest: 品川庄司)[073]091126 ひみつの嵐ちゃん!(VIP ROOM 釈由美子/特别企划SP 驱魔)[072]091119 ひみつの嵐ちゃん!(VIP ROOM suzanne/人体模特~以「红色」为元素的搭配~Guest: 宮迫博之藤本敏史)[071]091112 ひみつの嵐ちゃん!(VIP ROOM 広末涼子/人气嵐!差劲嵐!)[070]091105 ひみつの嵐ちゃん!(VIP ROOM 貫地谷しほり/人体模特~秋季夹克衫搭配~Guest: フットボールアワー)[069]091029 ひみつの嵐ちゃん!(VIP ROOM 黒谷友香/嵐10周年特別企划!欢迎光临嵐OK房)[068]091022 ひみつの嵐ちゃん!(VIP ROOM 明石家さんま/人体模特~秋季联谊的决胜服装~Guest: 有田哲平、宮川大輔、岡田圭右、設楽統、山根良顕)[067]090917 ひみつの嵐ちゃん!(VIP ROOM 井上真央/突击嵐的晚饭)[066]090910 ひみつの嵐ちゃん!(VIP ROOM 志田未来/地方料理Waiter)[065]090903 ひみつの嵐ちゃん!(VIP ROOM 石原さとみ/人体模特~想尝试下的冒险服装~)[064]090827 ひみつの嵐ちゃん!(VIP ROOM Becky/人气嵐!差劲嵐!SP)[063]090820 ひみつの嵐ちゃん!(真夏SP VIP ROOM 黒木瞳/人体模特SP~联谊时希望男方穿的服装~对决Guest: 藤本敏史、徳井義実、板倉俊之、たむらけんじ、村上健志)[062]090813 ひみつの嵐ちゃん!(VIP ROOM 上戸彩/皇家牛郎「松本潤vs後藤輝基」Guest:神田うの/巨星专用豪华之旅~榊原郁恵~)[061]090806 ひみつの嵐ちゃん!(VIP ROOM MARIE/人体模特~夏天穿的决胜T恤~Guest:SPEED)[060]090730 ひみつの嵐ちゃん!(VIP ROOM 米倉涼子/巨星专用豪华之旅~榊原郁恵~)[059]090723 ひみつの嵐ちゃん!(皇家牛郎「相葉雅紀vs細川茂樹」Guest:高畑敦子/地方料理Waiter/STRIKE LINE~バナナマン~)[058]090716 ひみつの嵐ちゃん!(皇家牛郎「二宮和也vs要潤」Guest:久本雅美/不知是福~優木まおみ~)[057]090709 ひみつの嵐ちゃん!(人气嵐!差劲嵐!/人体模特~去海边时希望男友穿的服装~)[056]090702 ひみつの嵐ちゃん!(人气嵐!差劲嵐!/嵐的5人连续演技挑战)[055]090625 ひみつの嵐ちゃん!(巨星专用豪华之旅~狩野英孝~/试用家电~叶美香+上原美優~)[054]090618 ひみつの嵐ちゃん!(不知是福~中川翔子~/巨星专用豪华之旅~はるな愛~)[053]090611 ひみつの嵐ちゃん!(试用家电~渡辺満里奈+misono~/人体模特~外出时希望男友穿的服装~)[052]090604 ひみつの嵐ちゃん!(STRIKE LINE~小池栄子+木下隆行~/巨星专用豪华之旅~DAIGO~)[051]090528 ひみつの嵐ちゃん!(人体模特~初次约会时希望男友穿的服装~/皇家牛郎「櫻井翔vs徳井義実」Guest:山村紅葉)[050]090521 ひみつの嵐ちゃん!(变性人意想不到的事件簿SP)[049]090514 ひみつの嵐ちゃん!(意想不到的结局BEST10/艺能界马里奥决战<後編>/日常生活耍帅企划)[048]090507 ひみつの嵐ちゃん!(意想不到的结局BEST10/艺能界马里奥决战<中編>)[047]090430 ひみつの嵐ちゃん!(呼唤奇迹的嵐SP/艺能界马里奥决战<前編>)[046]090423 ひみつの嵐ちゃん!(绝对不能错过的画面SP)[045]090319 ひみつの嵐ちゃん!(WBC緊急特番)[044]090312 ひみつの嵐ちゃん!(名人最新轶事大公开SP/自分説明書「大地真央」)[043]090305 ひみつの嵐ちゃん!(男女混战SP/自分説明書「深田恭子」)[042]090226 ひみつの嵐ちゃん!(世界了不起的歌声SP/自分説明書「柴田理恵」)[041]090219 ひみつの嵐ちゃん!(华丽的疯狂挑战者SP/日常生活耍帅企划/自分説明書「松平健」)[040]090212 ひみつの嵐ちゃん!(超级肥胖者SP/自分説明書「八嶋智人」)[039]090205 ひみつの嵐ちゃん!(美人New Half SP/自分説明書「DAIGO」)[038]090129 ひみつの嵐ちゃん!(高个子SP/中国巨人俱乐部/自分説明書「古閑美保」)[037]090122 ひみつの嵐ちゃん!(错觉图像SP/自分説明書「要潤」)[036]090115 ひみつの嵐ちゃん!(奇怪夫妇SP/自分説明書「壇れい」)★=====2008年=====★[035]081218 ひみつの嵐ちゃん!(嵐的忘年会SP)[034]081211 ひみつの嵐ちゃん!(世界的白痴尝试SP/穿T恤挑战世界纪录/自分説明書「竹中直人」)[033]081204 ひみつの嵐ちゃん!(奇迹的双胞胎大集合SP/自分説明書「真矢みき」)[032]081127 ひみつの嵐ちゃん!(亡命之徒SP/自分説明書「MEGUMI」)[031]081120 ひみつの嵐ちゃん!(整形美女SP/自分説明書「黒谷友香」)[030]081113 ひみつの嵐ちゃん!(超美New Half SP/自分説明書「高畑淳子」)[029]081106 ひみつの嵐ちゃん!(超肥胖者SP/自分説明書「佐田真由美」)[028]081030 ひみつの嵐ちゃん!(Super Kids SP/自分説明書「内田恭子」)[027]081023 ひみつの嵐ちゃん!(艺术家SP/自分説明書「绫濑遥」)[026]081016 ひみつの嵐ちゃん!(令人吃惊的表演SP/传说中的渔夫/自分説明書「戸田恵梨香」)[025]081009 ひみつの嵐ちゃん!(厉害美女SP/自分説明書「長谷川理恵」)[024]080925 ひみつの嵐ちゃん!(嵐の夏休み! in 日光<後編>/嵐的轮盘赌<New Half 篇>)。
日本文法子版(EMMA)
催促 说服、劝导 损坏、破坏、伤害 耸立 1.背向,2.违背、背叛 参与、参加 漂流、漂浮、飘浮 保持、维持 溺爱、娇养 花费、耗费 抓住、逮住 告诉、通知 发牢骚、唠叨 责难、责备、挑剔 完成、达到 1.堵塞、积压,2.耽误、延误 停止、停留 装糊涂、假装 带、伴随 叹息、叹气 渗出、渗入 拧、扭转 妒忌、羡慕 敲诈、勒索 粘、坚持 1.推敲、斟酌,2.锻炼 逃跑、逃避 面临、面对 攻占、侵占、夺取 骂 1.紧握,2.充分理解、掌握 1.照、映照,2.显眼 进展 努力、刻苦 1.跳、蹦,2.高涨
欺く 焦る 褪せる 促す 敬う
1.欺骗,2.赛过、胜过(甘言をもって欺 36 焦躁、急躁、着急 37 褪色、掉色 38 催促、促使 39 尊敬、敬重 40 厌烦、厌腻 41 蛊惑、煽动、怂恿 42 威胁、恐吓 43 1.佩带、携带,2.带有、含有 44 反省、自问 45 增大、增多 46 一致、符合 47 能实现、能如愿以偿 48 庇护、袒护、保护 49 1.过敏,2.着迷 50 锻炼 51 斟酌、考虑 52 不一致、有分歧 53 1.通过、钻过,2.潜水 54 1.打翻、弄翻,2.推翻、打倒 55 试试、试验一下 56 别扭、执拗 57 1.欺骗、欺瞒,2.蒙蔽、掩盖 58 凝、集中 59 因吃过苦头而不敢再尝试 60 遮挡、遮蔽 61 1.鸟叫,2.喋喋不休 62 寒冷、清澈 63 妨碍、阻碍 64 妨碍、阻碍 65 做完、完成 66 1.失败、失策,2.被解雇 67 爱慕、怀念、想念 68 枯萎、凋谢、瘪 69 1.废除,2.过时、不流行 70
71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
高性能螺旋絲攻,表面施以Tin塗層處理與特殊的螺紋設計,可 適用於各類型合金鋼材與非鐵合金材的盲孔加工使用,適合對 少 量 多 樣 性 材 料 加 工 。 螺 紋 部 採 YAMAWA獨 特 之 BLF構 型 設 計,可維持螺紋導程的穩定性與良好的排屑性。 大幅度降低切屑排出不良造成牙部崩損的問題。
鑄鐵用絲攻
超硬絲攻
X系列 高性能螺旋絲攻 高性能螺旋絲攻
高速加工用絲攻
低碳鋼
球墨鑄鐵 灰鑄鐵
P2
低碳鋼
球墨鑄鐵 灰鑄鐵
產品體系選用參考(鋼鐵材通孔用)
高硬度鋼
高硬度鋼
調質鋼 工具鋼 合金鋼 高碳鋼
中碳鋼
調質鋼 工具鋼 合金鋼 高碳鋼
中碳鋼
超硬絲攻 超硬絲攻
難削材先端絲攻
牙距同步進給範圍
+系列 高碳鋼用先端絲攻
製作尺寸
不 鋼用螺旋型先端絲攻 不 鋼用先端絲攻
專為不銹鋼、鉻鉬合金鋼等專用先端絲攻,對有黏性之材料具 有優良之切削性能。 ※SU+SL(通孔用)溝部採左旋設計,具有更佳的排屑性,可適用 於切削速度10~20m/min中高速度加工。
深孔加工用先端絲攻
深孔用絲攻主要適用於攻牙深度比絲攻外徑大2~3倍之深孔攻 牙,短螺紋設計能降低切削摩擦並且有良好的切屑排出能降低 切屑干涉造成絲攻折損的問題。
種 類
X系列螺旋型先端絲攻 高性能螺旋型先端絲攻 X系列高性能螺旋型先端絲攻
用途 特性
因應高速化與精密化要求的加工潮流,XSL系列產品採用新的 逆螺旋溝槽設計,較一般先端型絲攻有更優良的前排屑性能, 構型也採高精度與高剛性的DIN規範設計,可加工出精良的內螺 紋。 攻牙速度適用於切削速度(13~20m/min)的中高速度加工。 ※適用於具有牙距同步進給功能之數控機械與剛性夾持使用。
高硬度鋼
高硬度鋼
調質鋼 工具鋼 合金鋼 高碳鋼
中碳鋼
調質鋼 工具鋼 合金鋼 高碳鋼
中碳鋼
超硬絲攻 超硬絲攻
牙距同步進給範圍
難削材直溝絲攻
+系列
高碳鋼用螺旋絲攻
高碳鋼用螺旋絲攻
高速加工用 螺旋絲攻
螺旋絲攻 直溝絲攻
X系列 螺旋絲攻
+系列 螺旋絲攻
高速加工用絲攻
I系列直溝絲攻 I系列螺旋絲攻
軟鋼用螺旋絲攻
因應高速化與精密化要求的加工潮流,+PO先端絲攻針對螺紋 部要素進行重新設計並採用高精度之新構型,在中速度的嚴苛 的加工條件下,+PO先端絲攻更能顯現出螺紋精密度與壽命提 升效果。 ※適用於切削速度(8~16m/min)之中速度數控機械加工用。
P12
先端絲攻 螺旋型先端絲攻系列(通孔加工用)
商品記號
N+RZ升級版無溝絲攻,針對螺紋部要素進行重新設計並採用高 精度之新構型,能有效降低攻牙時的扭力與負荷,可加工出穩 定內螺紋精度,絲攻壽命也大幅提升。 (表面施以氧化處理)
R+V升級版鍍鈦無溝絲攻,針對螺紋部要素進行重新設計並採 用高精度之新構型,在高速度與嚴苛的加工條件下,仍能發揮 優異之性能。適用於各類材料加工。 (表面施以TiN處理)
高速加工用 螺旋絲攻
鋁合金 鋁壓鑄
輕合金用 直溝絲攻
鋁合金用 螺旋絲攻
超硬絲攻
合成塑脂用 直溝絲攻
高性能螺旋絲攻
高速加工用絲攻
X系列 高性能螺旋絲攻
高速加工用 超硬絲攻
牙距同步進給範圍
青銅 黃銅 鑄件 黃銅 銅
合成塑脂
產品體系選用參考(非鐵合金材通孔用)
鋁合金 鋁壓鑄 鋅合金
青銅 黃銅 鑄件 黃銅
銅
P13
難削材用先端絲攻 鈦合金用螺旋型先端絲攻 鎳合金用先端絲攻 長柄先端絲攻
難削材用絲攻主要適用於HRC30~45度之模具鋼、合金鋼等難 切材料加工,具有良好的耐磨耗性與切削性能。
專門針對鈦合金材之高強度與低熱傳導性特性所設計之絲攻, 擁有優良之切削性能,可對(HRC30~45)極難切削之材料進行加 工。 ※適用於具有牙距同步進給之加工機械使用。
針對鎳基合金等之抗熱蝕性及高溫時機械強度高的性能特性所 設計之絲攻,擁有優良之切削性與耐磨耗性。可對 (HRC30~45)極難切削之合金鋼、調質鋼等材料進行加工。 ※適用於具有牙距同步進給之加工機械使用。
LS-PO長柄絲攻長度共區分為6種: L-70mm、L-100mm、L-120mm、L-150mm L-200mm、L-250mm
無溝絲攻系列
商品記號
種 類
非鐵合金用無溝絲攻
鋼鐵合金用無溝絲攻
鍍鈦無溝絲攻
高碳鋼用無溝絲攻
免用油無溝絲攻
IT產業用無溝絲攻
不 鋼用無溝絲攻
用途 特性
製作尺寸
N+RS升級版無溝絲攻,針對螺紋部要素進行重新設計並採用高 精度之新構型,能有效降低攻牙時的扭力與負荷,可加工出穩 定內螺紋精度,絲攻壽命也大幅提升。 (表面施以滲氮硬化處理)
不鋼
不鋼
耐熱合金
不鋼
耐熱合金
鈦合金
鎳合金螺旋型先端絲攻 牙距同步進給範圍 鈦合金
鎳合金
難削材 不鋼
鎳合金先端絲攻
鎳合金先端絲攻
鎳合金
難削材 不鋼
+系列
不 鋼螺旋型先端絲攻
X系列
不 鋼先端絲攻
不 鋼螺旋型先端絲攻
高速加工用 絲攻
不鋼
P4
產品體系選用參考(非鐵合金材盲孔用)
鋁合金
鋁壓鑄
青銅 黃銅 鑄件 黃銅 銅 合成塑脂
+系列螺旋絲攻 X系列螺旋絲攻 高性能螺旋絲攻 X系列高性能螺旋絲攻
因應高速化與精密化要求的加工潮流,+SP螺絲攻針對螺紋部 要素進行重新設計並採用高精度之新構型,在中速度的嚴苛的 加工條件下,+SP螺旋絲攻更能顯現出螺紋精密度與壽命提升 效果。 ※適用於切削速度(8~16m/min)之中速度數控機械加工用。
新構型設計之螺旋絲攻,適合於一般鋼鐵材與非鐵合金盲孔加 工使用。SP螺旋絲攻攻牙時能將切屑由孔後方排出孔外,能避 免切屑堵塞於孔內造成絲攻損害。 (另公制粗牙尺寸有加大精度之產品可選擇) ※適用於切削速度(13m/min以下)各類型攻牙機械加工用。
標準型螺旋絲攻(氧化處理品)
新構型設計之螺旋絲攻表面氧化處理品,在加工鋼材時能降低 溶著(刀瘤)現象發生,可減少絲攻崩損。 (需使用油性切削油劑) ※適用於切削速度(13m/min以下)各類型攻牙機械加工用。
X系列高性能螺旋絲攻,表面施以Tin塗層處理與特殊的螺紋設 計,可適用於各類型合金鋼材與非鐵合金材的加工使用,適合 對少量多樣性材料加工。螺紋部採YAMAWA獨特之BLF構型設 計,可維持螺紋導程的穩定性與良好的排屑性,絲攻構型也採 高精度與高剛性的DIN規範構型設計,適用於15m/min以上之中 高速加工使用。 ※適用於具有牙距同步進給功能之數控機械與剛性夾持使用。
先端絲攻系列(通孔加工用)
商品記號
種 類
I系列先端絲攻 標準型先端絲攻 標準型先端絲攻(氧化處理品)
+系列先端絲攻
用途 特性
製作尺寸
IPO主要針對一般少量多樣化及產品試作等通孔螺紋加工使用。 產品表面施以氧化處理,最適合於SPC材、SS400材、低碳鋼 等的螺紋加工用。 ※適用於切削速度(5m/min以下)台式攻牙機或手持攻牙加工用。
不 鋼用螺旋絲攻 難削材不 鋼用螺旋絲攻
專為不銹鋼、鉻鉬合金鋼等加工之專用絲攻,具有優良之切削 功能。能有效降低攻牙時絲攻的負擔並加工出更優良的螺紋。 絲攻表面也施以氧化處理,可降低溶著問題的產生。
針對SUS316、317等難切削之不銹鋼與合金鋼、調質鋼等較難 切削之材料。SU2-SP螺紋部採BLF構型,除降低絲攻之切削阻 力外,更可降低切屑干涉造成絲攻折損的問題。絲攻表面也施 以氧化處理,可降低溶著問題的產生。
深孔用絲攻主要適用於攻牙深度比絲攻外徑大2~3倍之深孔攻 牙,短螺紋設計能降低切削摩擦並具有良好的切屑排出能降低 切屑干涉造成絲攻折損的問題。
專門針對鈦合金材之高強度與低熱傳導性特性所設計之絲攻, 擁有優良之切削性能,可對(HRC30~45)極難切削之材料進行加 工。 ※適用於具有牙距同步進給之加工機械使用。
低碳鋼
高碳鋼 中碳鋼
鍍鈦無溝絲攻
免用油無溝絲攻 高碳鋼用無溝絲攻
牙距同步進給範圍
泛用型 無溝絲攻
鋼鐵用無溝絲攻
高碳鋼用無溝絲攻 鍍鈦無溝絲攻
免用油無溝絲攻
低碳鋼
鍍鋅鋼板
產品體系選用參考(非鐵合金材無溝絲攻用)
鍍鋅鋼板
鋁合金
鋁壓鑄 鋅合金
青銅 黃銅 鑄件 黃銅
銅
非鐵合金用無溝絲攻
高碳鋼用無溝絲攻 鍍鈦無溝絲攻
針對鎳基合金等材料具高抗熱蝕性及高溫時機械強度高的特性 所設計之絲攻,擁有優良之切削性與耐磨耗性。並可針對硬度 (HRC30~45)之極難切削特殊合金鋼、調質鋼等材料進行加工。 ※適用於具有牙距同步進給之加工機械使用。
長柄螺旋絲攻
LS-SP長柄絲攻長度共區分為6ቤተ መጻሕፍቲ ባይዱ: L-70mm、L-100mm、L-120mm、L-150mm L-200mm、L-250mm
P11
螺旋絲攻系列(盲孔加工用)
商品記號
種 類
鋁合金用螺旋絲攻
用途 特性
製作尺寸
鋁合金專用螺旋絲攻主要針對鋁合金與鋁壓鑄合金使用,絲攻 中徑精度採用加大尺寸設計,表面施以滲氮硬化處理,對一般 鋁壓鑄材中含有Si成分具有更佳的耐磨耗性與耐久力。 (吃入部分成2種:1.5牙、2.5牙)
