00 PPT_Timken in China_CN_20120709
ANSI IEEE C57.110-1986

An American National Standard
IEEE Recommended Practice for Establishing Transformer Capability When Supplying Nonsinusoidal Load Currents
ii
Foreword
(This Foreword is not a part of ANSI/IEEE C57.110-1986, IEEE Recommended Practice for Establishing Transformer Capability When Supplying Nonsinusoidal Load Currents.)
© Copyright 1988 by The Institute of Electrical and Electronics Engineers, Inc 345 East 47th Street, New York, NY 10017, USA No part of this publication may be reproduced in any form, in an electronic retrieval system or otherwise, without the prior written permission of the publisher.
IEEE Standards documents are developed within the Technical Committees of the IEEE Societies and the Standards Coordinating Committees of the IEEE Standards Board. Members of the committees serve voluntarily and without compensation. They are not necessarily members of the Institute. The standards developed within IEEE represent a consensus of the broad expertise on the subject within the Institute as well as those activities outside of IEEE that have expressed an interest in participating in the development of the standard. Use of an IEEE Standard is wholly voluntary. The existence of an IEEE Standard does not imply that there are no other ways to produce, test, measure, purchase, market, or provide other goods and services related to the scope of the IEEE Standard. Furthermore, the viewpoint expressed at the time a standard is approved and issued is subject to change brought about through developments in the state of the art and comments received from users of the standard. Every IEEE Standard is subjected to review at least every five years for revision or reaffirmation. When a document is more than five years old and has not been reaffirmed, it is reasonable to conclude that its contents, although still of some value, do not wholly reflect the present state of the art. Users are cautioned to check to determine that they have the latest edition of any IEEE Standard. Comments for revision of IEEE Standards are welcome from any interested party, regardless of membership affiliation with IEEE. Suggestions for changes in documents should be in the form of a proposed change of text, together with appropriate supporting comments. Interpretations: Occasionally questions may arise regarding the meaning of portions of standards as they relate to specific applications. When the need for interpretations is brought to the attention of IEEE, the Institute will initiate action to prepare appropriate responses. Since IEEE Standards represent a consensus of all concerned interests, it is important to ensure that any interpretation has also received the concurrence of a balance of interests. For this reason IEEE and the members of its technical committees are not able to provide an instant response to interpretation requests except in those cases where the matter has previously received formal consideration. Comments on standards and requests for interpretations should be addressed to: Secretary, IEEE Standards Board 345 East 47th Street New York, NY 10017 USA IEEE Standards documents are adopted by the Institute of Electrical and Electronics Engineers without regard to whether their adoption may involve patents on articles, materials, or processes. Such adoption does not assume any liability to any patent owner, nor does it assume any obligation whatever to parties adopting the standards documents.
压力容器名录
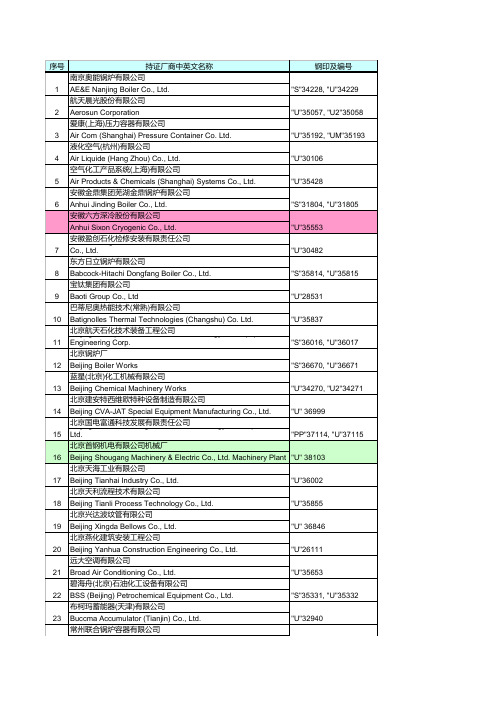
24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49
Chang Zhou Union Boiler & Pressure Vessel Co., Ltd. 常熟市高压容器制造有限公司 Changshu High-Pressure Vessel Manufacturing Co., Ltd. 常熟市医药化工设备总厂 & Chemical Equipment General Changshu Pharmaceutical Factory 常熟市上海飞奥压力容器制造有限公司 Vessel Manufacture Changshu Shanghai Fiorentini Pressure Co., Ltd. 常州锅炉有限公司 Changzhou Boiler Co., Ltd 常州市华立液压润滑设备有限公司 Changzhou Huali Hydraulic Lubrication Equipment Co., Ltd. 常州蓝翼飞机装备制造有限公司 Changzhou Lanyi Aircraft Equipment Manufacture Limited Company 常州市第二化工机械制造有限公司 Changzhou No. 2 Chemical Machinery Manufacture Co., Ltd 常州综研加热炉有限公司 Changzhou Soken Boiler Co., Ltd. 常州斯派克能源设备有限公司 Changzhou Spark Energy Equipment Co., Ltd 查特深冷工程系统(常州)有限公司 Chart Cryogenic Engineering Systems (Changzhou) Co., Ltd. 成都波威特斯空调技术有限公司 Chengdu Provides Air Conditioning Technology Co., Ltd. 成都天人压力容器厂 Chengdu Tianren Pressure Vessel Factory 中国第二重型机械集团(德阳) China Erzhong Group (Deyang) Heavy Industries Co., Ltd. 中国第二重型机械集团(德阳) China Erzhong Group (Deyang) Heavy Industries Co., Ltd. 中国第一重型机械集团公司 China First Heavy Industries 中国第一重型机械集团公司 China First Heavy Industries 中国华电工程(集团)有限公司 China Huadian Engineering Corp. 中国石油六建公司钦州港金属结构厂 China Petroleum 6th Construction Company 中国石油天然气第七建设公司机械设备工程分公司 China Petroleum 7th Construction Company
交叉滚柱轴环 CrossRollerRing

Lh
L N 60
Lh ∶工作寿命时间 N ∶每分钟转数
摇摆运动用
Lh
360 L 2 θ n° 60
Lh ∶使用寿命时间
∶摇摆角度
no ∶每分钟往返次数
(h) (min-1)
θ
(h) (度) (※参考右图) (min-1)
˞揺摆角度∶较小时,轨道圈和滚子的接触面难以形成油膜, 可能发生微动磨损。如要以这种条件使用时,请咨询THK。
滚柱接触长度
ℓ
ℓʼℓ
ℓ
ℓ
ℓ
配有间隔保持器
配有钢板保持器(传统型)
(2)在传统型号中,如下图所示,外圈侧和内环侧的负荷区域相对于滚柱长度的中央为不对称结构。因 而,随著负荷的增大,力矩也增大,引起端面接触。此外,由于摩擦阻力增大,从而不能进行平稳的旋 转运动,磨损也将加快。
端面接触
负荷区对称 配有间隔保持器
【大幅度地提高了刚性(比传统型号提高3~4倍)】
与使用双列薄形角接触球轴承不同,由于滚柱为交叉排列,因此只用1个交叉滚柱轴环就可承受各个方 向的负荷。并且与传统型号相比,刚性被提高了3~4倍。
梯度角∶rad 双列角接触球轴承
力矩刚性图 ʷ
33"#
(N) (N) (N) (N·mm) (X0=1) (Y0=0.44) (mm)
径向载荷 (Fr)
力矩(M)
轴向载荷 (Fa) 力矩 (M)
C0 fS P0
fS ∶静态安全系数 C0 ∶基本静额定载荷 P0 ∶静态等价径向载荷
(参照表3) (N) (N)
【静态等价径向载荷P0】
交叉滚柱轴环的静态等价径向载荷可按下式计算。
FMC Weco 由壬 Wing Union

I2
Flowline Products and Services
Unsurpassed Quality
FMC’s fluid control quality system has been surveyed and approved by DNV and meets ISO 9001 and European Pressure Equipment Directive 97/23/CE. Most products are supplied with the CE marking. Chiksan and Weco products also can be supplied with both type and case approval from DNV, Lloyds, ABS, GGTN, and others. Products for sour gas service meet NACE MR-01-75 and API RP-14-E. Complete material certification and traceability are also available.
ExperLeabharlann enced, Knowledgeable, Productive People
FMC’s global fluid control team is structured around top flowline professionals – individuals who understand your business and are dedicated to meeting your needs. The management, engineering, and sales support staff are among the most experienced in the oil and gas industry. Their knowledge and industry expertise show up in the quality of products and services delivered to you.
派克Parker流体连接件中国区产品手册 上

Parkrimp 软管扣压机
派克Parkrimp扣压设备使软管的扣压能 够更加方便快捷。
使用Parkrimp设备来扣压派克的不剥胶 软管,扣压前不需要剥去软管外胶层, 整个装配过程只需要几分钟就能完成。 更重要的是,Parkrimp在使用过程中不 需要对任何仪器进行设置,操作过程简 单方便,易于上手,不需要专门的培训 即可使用。
派克青岛实验室
工厂认证
软管和接头的生产都需要满足 工业标准的要求。派克青岛产 品的生产达到甚至超过了以下 标准: • SAE(美国汽车工程师协会) • EN(欧洲标准) • DIN(德国工业标准) • ISO(国际标准化组织) • MA(煤矿矿用产品安全标志
证书)
4
中国常用流体连接件产品概览
液压软管及其接头
不断创新
我们的使命是:持续改进,不断创新,朝着更 小、更轻、更节能、更可靠的目标前进,与客 户成为合作伙伴,共同进步。
2
关于派克中国
派克中国总部,位于上海市浦东新区金桥开发区
派克汉尼汾在中国的发展 开始于20世纪80年代,是 改革开放后最早在中国建 立合资公司的美国工业公 司之一。如今,公司产品 已经覆盖了包括能源、轨 道交通、流体处理、行走 液压、海洋开发、工厂自 动化和冶金等超过50个市 场领域。公司总部位于上 海,在成都、北京、广州 等地设有销售办事处。
派克致力于为您提供一个更高压 力、更易安装、无需维护的无泄 漏系统。
诊断设备
使用SensoControl® 产品对系统 进行监测、分析并且优化
从数字压力表到多 通道测试仪,派克的 SensoControl® 便携式诊 断设备可以检测并 记录液压系统的 各个参数,如压 力、温度、流 量、转速等;同 时能够捕捉到瞬 间压力峰值及流量 峰值,有助于早期故障 识别和分析,帮助优化行 走机械及工业液压系统。
CCMT2012“数控机床专项成果及应用展”

( )积极打造完整产业链 ,推进 行业 结构调整 1
研 发完 成 了一批 进 口量 大 、市场 需 求面 广 的加 工 中心和 数控车床 ,根据 已验 收课题 的第 三方检测机
控 机床 与基础制造装备进行 了全面部署 。
本刊编 辑部
E i ra e t o d t i l p . f MT o D &MT
为 了 更
工程 l 项 ,创 新能 力平 台建设 l项 ,用户 工艺应用试 3 7
验 研 究4 。 项
好 地宣 传 高档 数 控机 床重 大
专 项 成 果 , 推
C M 2 1 数控机床专项 成果及应用展 由工业和信 C T0 2
刀具等 产业链 中的薄弱环节进 行攻关 ,并开始批量配 套应用 。在高档数控 系统研发 方面 ,由于 掌握 了先进 的硬件 设计和焊装 技术 ,两种 典型高档数控 系统新产 品H C 4 包 括应用 国产C U N 8 8( P 的系统 )和G C 0 N 6 的系统
的基 础 。
国务 院发 布 的 《 国家 中长 期科 学 和 技术 发 展 纲
要》 ( 0 6 2 2 )确定 “ 20—00 高档数控机床 与基 础制造 装 备 ”为l 个 国家科技重大专项之 一。 6 2 0 年 ,工业和信 息化 部牵头组织 实施 “ 09 高档 数 控 机床与基 础制造装 备 ”科技 重大专项 。围绕航空 航
C 2 ‘ MT 0 ‘ C 1 2 数控机 床专项成果及应
用展”
“ci e etadapctn xitn f a r c ne n cnl y r r ” C T 02 A h vm n plao h i j i c d eho g o a aC M 2 1 e sn i i e bi oM o S e a T o o Pgm t
攻丝-术语和应用
外螺纹(螺栓)
保密资料,仅供内部使用
© 2013 Kennametal Inc. l All rights reserved. l Proprietary and Confidential l 6 of 110
螺纹 – 术语
螺纹直径
中径最为重要,因为它控制 所有螺纹组装的配合与强度
中径 外螺纹 (螺栓 )
•
保密资料,仅供内部使用
© 2013 Kennametal Inc. l All rights reserved. l Proprietary and Confidential l 9 of 110
螺纹命名
螺纹命名
1/4-20 UNC-2B (Unified Inch) (统一英制 )
标称尺寸
(每英寸螺 纹数 ) 螺距
丝锥槽型 – 排屑槽
基本设计特点
刃背宽度
核心直径(扭 转强度) 排屑槽间距(容屑 和润滑)
这些设计特点必须保持平衡,才能提供适当的切削,切屑控 制,润滑和扭转强度!
保密资料,仅供内部使用
© 2013 Kennametal Inc. l All rights reserved. l Proprietary and Confidential l 22 of 110
螺纹命名
统一化系列的实例
以下为美国统一标准系列 :
1/4-20 UNC
1
粗齿
2
1/4-28 UNF
细齿 超细齿 专用齿
1/4-32 UNEF
1/4-40 UNS
保密资料,仅供内部使用
© 2013 Kennametal Inc. l All rights reserved. l Proprietary and Confidential l 11 of 110
搅拌摩擦焊的工艺参数
Trans. Nonferrous Met. Soc. China 22(2012) 1064í1072Correlation between welding and hardening parameters offriction stir welded joints of 2017 aluminum alloyHassen BOUZAIENE, Mohamed-Ali REZGUI, Mahfoudh AYADI, Ali ZGHALResearch Unit in Solid Mechanics, Structures and Technological Development (99-UR11-46),Higher School of Sciences and Techniques of Tunis, TunisiaReceived 7 September 2011; accepted 1 January 2011Abstract: An experimental study was undertaken to express the hardening Swift law according to friction stir welding (FSW) aluminum alloy 2017. Tensile tests of welded joints were run in accordance with face centered composite design. Two types of identified models based on least square method and response surface method were used to assess the contribution of FSW independent factors on the hardening parameters. These models were introduced into finite-element code “Abaqus” to simulate tensile tests of welded joints. The relative average deviation criterion, between the experimental data and the numerical simulations of tension-elongation of tensile tests, shows good agreement between the experimental results and the predicted hardening models. These results can be used to perform multi-criteria optimization for carrying out specific welds or conducting numerical simulation of plastic deformation of forming process of FSW parts such as hydroforming, bending and forging.Key words: friction stir welding; response surface methodology; face centered central composite design; hardening; simulation; relative average deviation criterion1 IntroductionFriction stir welding (FSW) is initially invented and patented at the Welding Institute, Cambridge, United Kingdom (TWI) in 1991 [1] to improve welded joint quality of aluminum alloys. FSW is a solid state joining process which was therefore developed systematically for material difficult to weld and then extended to dissimilar material welding [2], and underwater welding [3]. It is a continuous and autogenously process. It makes use of a rotating tool pin moving along the joint interface and a tool shoulder applying a severe plastic deformation [4].The process is completely mechanical, therefore welding operation and weld energy are accurately controlled. B asing on the same welding parameters, welding joint quality is similar from a weld to another.Approximate models show that FSW could be successfully modeled as a forging and extrusion process [5]. The plastic deformation field in FSW is compared with that in metal cutting [6í8]. The predominant deformation during FSW, particularly in vicinities of thetool, is expected to be simple shear, and parallel to the tool surface [9]. When the workpiece material sticks to the tool, heat is generated at the tool/workpiece contact due to shear deformation. The material becomes in paste state favoring the stirring process within the thermomechanically affected zone, causing a large plastic deformation which alters micro and macro structure and changes properties in polycrystalline materials [10].The development of the mechanical behavior model, of heterogeneous structure of the welded zone, is based on a composite material approach, therefore it must takes into account material properties associated with the different welded regions [11]. The global mechanical behavior of FSW joint was studied through the measurement of stress strain performed in transverse [12,13] and longitudinal [14] directions compared with the weld direction. Finite element models were also developed to study the flow patterns and the residual stresses in FSW [15]. B ased on all these models, numerical simulations were performed in order to investigate the effects of welding parameters and tool geometry on welded material behaviors [16] to predict the feasibility of the process on various shape parts [17].Corresponding author: Mohamed-Ali REZGUI; E-mail: mohamedali.rezgui@ DOI: 10.1016/S1003-6326(11)61284-3Hassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í1072 1065 However, the majority of optimization studies of theFSW process were carried out without being connectedto FSW parameters.In the present study, from experimental andmodeling standpoint, the mechanical behavior of FSWaluminum alloy 2017 was examined by performingtensile tests in longitudinal direction compared with theweld direction. It is a matter of identifying the materialparameters of Swift hardening law [18] according to theFSW parameters, so mechanical properties could bepredicted and optimized under FSW operating conditions.The strategy carried out rests on the response surfacemethod (RSM) involving a face centered centralcomposite design to fit an empirical models of materialparameters of Swift hardening law. RSM is a collectionof mathematical and statistical technique, useful formodeling and analysis problems in which response ofinterest is influenced by several variables; its objective isto optimize this response [19]. The diagnostic checkingtests provided by the analysis of variance (ANOV A) suchas sequential F-test, Lack-of-Fit (LoF) test, coefficient ofdetermination (R2), adjusted coefficient of determination(2adjR) are used to select the adequacy models [20].2 Experimental2.1 Welding processThe aluminum alloy 2017 chosen for investigationhas good mechanical characteristics (Table 1), excellentmachinability and formability, and is mostly used ingeneral mechanics applications from high strengthsuitable for heavy-duty structural parts.Table 1 Mechanical properties of aluminum alloy 2017Ultimate tensile strength/MPaYieldstrength/MPaElongation/%Vickershardness427 276 22 118 The experimental set up used in this study was designed in Kef Institute of Technology (Tunisia). A 7.5 kW powered universal mill (Momac model) with 5 to 1700 r/min and welding feed rate ranging from 16 to 1080 mm/min was used. Aluminum alloy 2017 plate of6 mm in thickness was cut and machined into rectangular welding samples of 250 mm×90 mm. Welding test was performed using two samples in butt-configuration, in contact along their larger edge, fixed on a metal frame which was clamped on the machine milling table.To ensure the repeatability of the FSW process, clamping torque and flatness surface of the plates to be welded are controlled for each welding test. At the end of welding operation, around 80 s are respected before the withdrawal of the tool and the extracting of the welded parts. In this experimental study, we purpose to screen theeffects of three operating factors, i.e. tool rotational speed N, tool welding feed F and diameter ratio r, on hardening parameters from Swift’s hardening law such as strength coefficient (k), initial yield strain (İ0) and hardening exponent (n). The ratio (r=d/D) of pin diameter (d) to shoulder diameter (D), is intended to optimize the tool geometry [21í23]. The welding tool is manufactured from a high alloy steel (Fig. 1).Fig. 1 FSW tool geometry (mm)Preliminary welding tests were performed to identify both higher and lower levels of each considered factors. These limits are fixed from visual inspections of the external morphology and cross sections of the welded joints with no macroscopic defects such as surface irregularities, excessive flash, and lack of penetration or surface-open tunnels. However, among these limits one is not sure to have a safe welded joint so often, but they show great potential on defect avoidance. Figure 2 shows some external macroscopic defects observed beyond the limit levels established for each factor. Table 2 lists the processing factors as well as levels assigned to each, and Table 3 shows the fixed levels for other factors needed to success the welding tests.A face centered central composite design, which comes under the RSM approach, with three factors was used to characterize the nature of the welded joints by determining hardening parameters. In this design the star points are at the center of each face of the factorial space (Į=±1), all factors are run at three levels, which are í1, 0, +1 in term of the coded values (Table 4). The experiment plan has been run in random way to avoid systematic errors.2.2 Tensile testsThe tensile tests are performed on a Testometric’s universal testing machines FSí300 kN. The tensile test specimens (ASME E8Mí04) proposed for characterizing the mechanical behavior of the FSW joint, were cut inHassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í10721066Fig. 2 Types of macroscopic defectsTable 2 Levels for operating parameters for FSW processFactorLow level (í1) Center point (0) High level(+1)N /(r·min í1) 653 910 1280 F /(mm·min í1)67 86 109r /%33 39 44Table 3 Welding parametersPin height/ mm Shoulder diameter/ mm Small diameter pin/mm Tool’s inclination angle/(°) Penetrationdepth of shoulder/mm5.3 18 4 30.78longitudinal direction compared with the weld direction, so that active zone is enclosed in the central weld zone (Fig. 3). Figure 4 shows the tensile specimens after fracture.Ultimately, it is a matter of experimental evaluation of hardening parameters of the behavior of FSW joints (k , İ0, n ) according to Swift’s hardening law:n k )(p 0H H V (1)These parameters are required to identify the plastic deformation aptitude of the FSW joints. They are also needed for numerical simulations of forming operations on welded plates. The hardening parameters have been calculated by least square method (LSM) from the stressüstrain curves data. Table 4 shows the experimental design as well as dataset performance characteristics according to the FSW parameters of aluminum Alloy 2017.3 Experimental results3.1 Development of mathematical modelsAlthough the basic principles of FSW are very simple, it involves complex phenomena related to thermo-mechanical and metallurgical transformation that causes strong microstructural heterogeneities in the welded zone. From an energy standpoint, welding process is generated by converting mechanical energy provided by FSW tool into other types of energy such as heat, plastic deformation and microstructural transformations. The nonlinear character of these different dissipation forms can justify research for nonlinear prediction models whose accuracy generally depends on the order of the models relating the responses to welding parameters. For this reason, we chose the RSM which is helpful in developing a suitable approximation for the true functional relationships between quantitative factors (x 1, x 2, Ă, x k ) and the response surface or response functions Y (k , İ0, n ) that may characterize the nature of the welded joints as follows:r 21),,,(e x x x f Y k (2)Hassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í10721067Table 4 Face centered central composite design for FSW of aluminum alloy 2017Factors levelCoded Actual Hardening parameterTypeStandard orderN F r N /(r·m í1)F /(mm·min í1)r /% k /MPan İ0/%1 í1 í1 í165367 33629.7 0.3296 0.00202 1 í1 í1 1280 67 33 654.7 0.4514 0.0035 3 í1 1 í1 653109 33 587.8 0.3712 0.0025 4 1 1 í1 1280 109 33 689.2 0.4856 0.00555 í1 í1 1 653 67 44 642.3 0.4524 0.00256 1 í1 1128067 44 218.6 0.2447 0.0015 7 í1 1 1 653 109 44 685.5 0.4885 0.0035 Factorialdesign8 1 1 1 1280 109 44 332.5 0.3405 0.00209 0 0 0 91086 39 624.9 0.4257 0.0025 10 0 0 0 910 86 39 639.9 0.4292 0.0025 11 0 0 0 910 86 39 640.9 0.4011 0.0020 Center point12 0 0 0 910 86 39 598.6 0.3960 0.0023 13 í1 0 0 653 86 39 690.6 0.4748 0.0027 14 1 0 0 128086 39 505.6 0.3909 0.0030 15 0 í1 091067 39499 0.3317 0.001716 0 1 0 910 109 39 545.6 0.4157 0.0026 17 0 0 í1 910 86 33 672.1 0.4385 0.0027 Star point18 0 019108644 509.7 0.41750.0019Fig. 3 Tensile test specimens (ASME E8Mí04) cut in longitudinal direction compared with weld direction (mm)Fig. 4 Tensile specimens after fractureThe residual error term (e r ) measures theexperimental errors. Such relationship was developed as quadratic polynomial under multiple regression form [19,20]:¦¦¦ r 20e x x b x b x b b Y j i ij i ii i i (3)where b 0 is an intercept or the average of response; b i , b ii , and b ij represent regression coefficients. For the three factors, the selected polynomial could be expressed as:2332222113210r b F b N b r b F b N b b YFr b Nr b NF b 231312 (4)In applying the RSM, the independent variable Y was viewed as surface to which a mathematical model was fitted. The adequacy of the developed model was tested using the analysis of variance (ANOV A) which quantifies the amount of variation in a process and determines if it is significant or is caused by random noise.3.2 Mathematic model of hardening parametersTable 5 lists the coefficients of the best linear regression models. All selected parameters (N , F , r ) for k and İ0 are statistically significant (P-value less than 0.05) at the 95% confidence level. However, for the response n , the term b 3r having a P-value=0.0654>0.05 is not statistically significant at the 95% confidence level even though the term b 13Nr is statistically significant. Consequently, b 3(r ) is kept in the model to improve the Lack-of-Fit test (Table 6). Furthermore, only theHassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í10721068Table 5 Coefficients of regression models for hardening parametersStrength coefficient (k) Hardeningcoefficient(n) Initial yield strain (İ0) CoefficientEst. SEP-value Est SE P-valueEst/10í4 SE/10í4 P-value b0 610.39,48<10í4 0.422 0.0073 <10-4 22.8 1.010 <10-4 b1 í83.58.48<10í4 í0.020 0.0065 0.0091 2.30 0.912 0.0267 b2 19.68.480.0410.0290.00650.00084.900.9120.0002b3 í84.58.48<10í4 í0.013 0.0065 0.0654 í4.80 0.912 0.0002 b11 5.561.3670.0009b22 í61.812.720.0005í0.0310.00980.009b33b12b13 í112.99.48<10í4 í0.074 0.0073 <10-4 -8,75 1.010 <10-4 b23R2 95.90% 92.38% 92.84%2adjR 94.19% 89.21% 89.86% SE of est. 30.7 0.021 2.9×10í4Est: Estimate; SE: Standard Error; SE of est.: Standard error of estimateTable 6 ANOV A for hardening parametersk n İ0Source of variationSS Df P-Value SS Df P-Value SS/10í7 Df P-Value Model 263946.0 5 <10í4 0.062357 5 <10í4 129.324 5 <10í4Residual 11296.4 12 0.005140912 9.97 12Lack-of-Fit 10130.4 9 0.2065 0.00428669 0.3678 8.295 9 0.3723 Pure error 1166.07 3 0.0008543 3 1.675 3 Total correction 275243.017 0.06749817 139.294 17 DW-value 1.31 1.42 2.26DW: Durbin-Watson statistic; SS: Sum of squares; D f: Degree of freedominteraction (Nír) is statistically significant on the three responses (Fig. 5). According to the adjusted R2 statistic, the selected models explain 94.19%, 89.21% and 89.86% of the variability in k, n and İ0 respectively.The ANOV A (Table 6) for the hardening parameter shows that all models (k, n, İ0) represent statistically significant relationships between the variables in each model at the 99% confidence level (P-value<10í4). The Lack-of-Fit test confirms that these models (k, n, İ0) are adequate to describe the observed data (P-value>0.05) at the 95% confidence level. The DW statistic test indicates that there is probably not any serious autocorrelation in their residuals (DW-value>1.4). The normal probability plots of the residuals suggest that the error terms, for these models, are indeed normally distributed (Fig. 6). The response surface models in terms of coded variables (Eqs. (5)í(7)) are shown in Fig. 7.k=610.3–83.5 N+19.6 F–84.5 r –61.8 F2–112.9 Nr(5) n=0.422–0.020 N+0.029 F–0.013 r–0.031 F2–0.074 Nr(6) İ0=22.8+2.3 N+4.90 F–4.80 r+5.56 N2–8.75 Nr(7) Fig. 5 Interaction plots of Nír (rotational speedídiameter ratio): (a) Strength coefficient k; (b) Hardening coefficient n;(c) Initial yield strain İ0Hassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í1072 1069Fig. 6 Normal probability plots for residual: (a) Strength coefficient k; (b) Hardening coefficient n; (c) Initial yield strain İ04 Validation of identified modelsValidation tests of the identified models were performed through comparative study between the experimental models (EM) of tensile tests and the computed responses given by numerical simulations of the same tests (Fig. 8). The computed responses, expressed in the form of tension and elongation, wereFig. 7 Response surfaces plots: (a) Strength coefficient k;(b) Hardening coefficient n; (c) Initial yield strain İ0 established by examining welded joints having an elastoplastic behavior in accordance with the Swift hardening law (Eq. (1)). These computed responses were deduced from the numerical simulations using the finite element code Abaqus/Implicit, in which the introduced elastoplastic behavior was obtained from the least square hardening models (LSHM) (Table 4) and the response surface hardening models (RSHM) (Table 5). The highest deviations (<10%), between EM and computed response, were recorded with the RSHM. Increasing deviations, as shown in Fig. 8, is due to the effect of combining damage with plastic strains accumulatedHassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í10721070Fig. 8 Relationship between tension and elongation: Confrontation between experimental model (EM), and computed responses (LSHM, RSHM) for three experimental testsduring the onset of localized necking.The relative average deviation criterion (EM/LSHM ]) between the experimental data and the numerical predictions of tensions, was used to assess the quality of the identified models.¦¸¸¹·¨¨©§'' '2exp num exp exp/num )()()(1i i i L F L F L F N] (8)where N is the number of experimental measurements,F exp (ǻL i ) and F num (ǻL i ) are respectively the experimental and predicated tensions relating to the i-th elongation ǻL i . Figure 9 illustrates that the relative average deviation of EM/LSHM (EM/LSHM ]) ranges between 1.64% and 6.75% while the relative average deviation of EM/RSHM (EM/RSHM ]) ranges between 4.52% and 9.32%.Fig. 9 Distribution of relative average deviations for most representative experimental testsFor the deviation within limits fluctuating between 4.52% and 6.75% the estimated models (LSHM and RSHM) are comparable. This applies particularly to welded joints characterized by a strength coefficient (k ), ranging from 520 to 610 MPa and a hardening exponent (n ) ranging between 0.30 and 0.45.5 DiscussionIn this study we evaluated, using RSM, the effect of FSW parameters such as tool rotational speed, welding feed rate and diameter ratio of pin to shoulder on the plastic deformation aptitudes of welded joints. The performed analysis highlights the incontestable significant effects of rotational speed (N ), welding feed rate (F ) and the interaction (Nír ) between rotational speed and diameters ratio on hardening parameters (k , n , İ0) according to Swift law. The established models show that tool diameter ratio has a linear effect only on (k ) and (İ0), it does not have any quadratic effect. They also show that rotational speed has a quadratic effect solely on (İ0); while welding feed rate has a quadratic effect on both (k ) and (n ).In addition, numerical simulation of tensile tests of welded joints has been made possible through the predictive models (LSHM and RSHM) of Swift’s hardening parameters. To judge whether the models represent correctly the data, a comparative study between the experimental response and the computed response, expressed in terms of tension-elongation, was carried out. It was found that the relative average deviation betweenHassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í1072 1071experimental model and numerical models is less than 9.5% in all cases.Moreover, correlation between welding and hardening parameters provided has many benefits. The correlation relationships can solve inverse problem relating to optimal choice of parameters linked up with the desired welded joints properties to produce welds having tailor-made mechanical properties. The correlation predictions offer the possibility to identify the behavior of friction stir welded joints necessary for finite element simulations of various forming processes while minimizing experimental cost and time. Ultimately, understanding correlations can be useful for studies on reliability of welded assemblies in service life expectancy.6 Conclusions1) Rotational speed and welding feed rate are the factors that have greater influence on hardening parameters (k, n, İ0), followed by diameter ratio that has no influence on the hardening coefficient (n).2) The numerical models RSHM were compared with those through LSHM and confronted to the experimental results. Indeed, within the limit of a relative average deviation of about 9.3%, between the experimental model and numerical models expressed in terms of tension-elongation, the validity of these models is acceptable.3) The predictive models of work-hardening coefficients, established taking into account the FSW parameters, have made possible the numerical simulation of tensile tests of FSW joints. These results can be used to perform multi-criteria optimization for producing welds with specific mechanical properties or conducting numerical simulation of plastic deformation of forming process of friction stir welded parts such as hydroforming, bending and forging.References[1]THOMAS W M, NICHOLAS E D, NEEDHAM J C, MURCH M G,TEMPLE-SMITH P, DAWES C J. Friction stir butt welding,PCT/GB92/ 02203 [P]. 1991.[2]XUE P, NI D R, WANG D, XIAO B L, MA Z Y. Effect of friction stirwelding parameters on the microstructure and mechanical propertiesof the dissimilar AlíCu joints[J]. Materials Science and EngineeringA, 2011, 528: 4683í4689.[3]LIU H J, ZHANG H J, YU L. Effect of welding speed onmicrostructures and mechanical properties of underwater friction stirwelded 2219 aluminum alloy [J]. Materials and Design, 2011, 32:1548í1553.[4]MISHRA R S, MA Z Y. Friction stir welding and processing [J].Materials Science and Engineering R, 2005, 50: 1í78.[5]ARB EGAST W J. A flow-partitioned deformation zone model fordefect formation during friction stir welding [J]. Scripta Materialia,2008, 58: 372í376.[6]LEWIS N P. Metal cutting theory and friction stir welding tooldesign [M]. NASA Faculty Fellowship Program Marshall SpaceFlight Center, University of ALABAMA, NASA/MSFC Directorate:Engineering (ED-33), 2002.[7]ARB EGAST W J. Modeling friction stir welding joining as ametalworking process, hot deformation of aluminum alloys III [C].San Diego: TMS Annual Meeting, 2003: 313í327.[8]ARTHUR C N Jr. Metal flow in friction stir welding [R]. NASAmarshall space flight center, EM30. Huntsville, AL 35812.[9]FONDA R W, B INGERT J F, COLLIGAN K J. Development ofgrain structure during friction stir welding [J]. Scripta Materialia,2004, 51: 243í248.[10]NANDAN R, DEBROY T, BHADESHIA H K D H. Recent advancesin friction stir weldingüProcess, weldment structure and properties[J]. Progress in Materials Science, 2008, 53: 980í1023.[11]LOCKWOOD W D, TOMAZ B, REYNOLDS A P. Mechanicalresponse of friction stir welded AA2024: Experiment and modeling[J]. Materials Science and Engineering A, 2002, 323: 348í353. [12]SALEM H G, REYNOLDS A P, LYONS J S. Microstructure andretention of superplasticity of friction stir welded superplastic 2095sheet [J]. Scripta Materialia, 2002, 46: 337í342.[13]LOCKWOOD W D, REYNOLDS A P. Simulation of the globalresponse of a friction stir weld using local constitutive behavior [J].Materials Science and Engineering A, 2003, 339: 35í42.[14]SUTTON M A, YANG B, REYNOLDS A P, YAN J. B andedmicrostructure in 2024–T351 and 2524-T351 aluminum friction stirwelds, Part II. Mechanical characterization [J]. Materials Science andEngineering A, 2004, 364: 66í74.[15]ZHANG H W, ZHANG Z, CHEN J T. The finite element simulationof the friction stir welding process [J]. Materials Science andEngineering A, 2005, 403: 340í348.[16]ZHANG Z, ZHANG H W. Numerical studies on controlling ofprocess parameters in friction stir welding [J]. Journal of MaterialsProcessing Technology, 2009, 209: 241í270.[17]B UFFA G, FRATINI L, SHIVPURI R. Finite element studies onfriction stir welding processes of tailored blanks [J]. Computers andStructures, 2008, 86: 181í189.[18]SWIFT H W. Plastic instability under plane stress [J]. Journal of theMechanics and Physics of Solids, 1952, 1: 1í18.[19]MONTGOMERY D C. Design and analysis of experiments [M].Fifth Edition. New York: John Wiley & Sons, 2001: 684.[20]MYERS R H, MONTGOMERY D C, ANDERSON-COOK C M.Response surface methodology: Process and product optimizationusing designed experiment [M]. 3rd Edition. New York: John Wiley& Sons, 2009: 680.[21]VIJAY S J, MURUGAN N. Influence of tool pin profile on themetallurgical and mechanical properties of friction stir welded Al–10% TiB2 metal matrix composite [J]. Materials and Design,2010, 31: 3585í3589.[22]ELANGOV AN K, BALASUBRAMANIAN V. Influences of tool pinprofile and tool shoulder diameter on the formation of friction stirprocessing zone in AA6061 aluminum alloy [J]. Materials andDesign, 2008, 29: 362í373.[23]PALANIVEL R, KOSHY MATHEWS P, MURUGAN N.Development of mathematical model to predict the mechanicalproperties of friction stir welded AA6351 aluminum alloy [J]. Journalof Engineering Science and Technology Review, 2011, 4(1): 25í31.Hassen BOUZAIENE, et al/Trans. Nonferrous Met. Soc. China 22(2012) 1064í107210722017䪱 䞥 ⛞ ⛞⹀ ⱘ ㋏Hassen BOUZAIENE, Mohamed-Ali REZGUI, Mahfoudh AYADI, Ali ZGHALBesearch Unit in Solid Mechanics, Structures and Technological Development (99-UR11-46),Higher School of Sciences and Techniques of Tunis, Tunisia㽕˖ 2017䪱 䞥䖯㸠 ⛞ ˈ㸼䗄Swift⹀ 㾘 DŽ䞛⫼䴶 䆒䅵 ⊩䖯㸠⛞ ⱘ Ԍ 偠䆒䅵DŽ䞛⫼ Ѣ ѠЬ⊩ 䴶⊩ⱘ2⾡ 䆘Ԅ ⛞ ⛞ ㋴ ⹀ ⱘ DŽ䞛⫼ 䰤 Abaqus ⛞ Ԍ⌟䆩㒧 DŽⳌ 㒧 㸼 ˈ 偠㒧 㒧 䕗 DŽ䖭ѯ㒧 㛑⫼Ѣ 偠 Ⳃ Ӭ ˈ 㸠 ԧ⛞ ⛞ 䳊ӊ 䖛Ё ⱘ ˈ ⎆ ǃ 䬏䗴DŽ䬂䆡˖ ⛞ ˗ 䴶 ⊩˗䴶 Ё 䆒䅵˗⹀ ˗ ˗Ⳍ(Edited b y LI Xiang-qun)。
plant and soil
REGULAR ARTICLEEffects of NO3−-N on the growth and salinity tolerance of Tamarix laxa WilldXiaodong Ding&Changyan Tian&Shirong Zhang&Jie Song&Fusuo Zhang&Guohua Mi&Gu FengReceived:20October2009/Accepted:8November2009/Published online:5December2009#Springer Science+Business Media B.V.2009Abstract The influence of NO3−-N on growth and osmotic adjustment was studied in Tamarix laxa Willd.,a halophyte with salt glands on its twigs. Seedlings of xa Willd.were exposed to1mM (control)or300mM NaCl,with0.05,1or 10mM NO3−-N for24days.The relative growth rate of seedlings at300mM NaCl was lower than that of control plants at all NO3−-N levels,but the concentrations of organic N and total N in the twigs did not differ between the two NaCl treatments. Increasing NO3−supply under300mM NaCl im-proved the growth of xa,indicating that NO3−played positive roles in improving salt resistance of the plant.The twigs of xa Willd.accumulated mainly inorganic ions,especially Na+and Cl−,to lower osmotic potential(Ψs):the contributions of Na+ and Cl−toΨs were estimated at31%and27% respectively,at the highest levels of supply of both NaCl and NO3−-N.The estimated contribution of NO3−-N toΨs was as high as20%in the twigs in these conditions,indicating that NO3−was also involved in osmotic adjustment in the twigs.Further-more,increases in tissue NO3−were accompanied by decreases in tissue Cl−and proline under 300mM NaCl.The estimated contribution of proline toΨs declined as with NO3−-N supply increased from 1to10mM,while the contributions of nitrate toΨs were enhanced under300mM NaCl.This suggested that higher accumulation of nitrate in the vacuole alleviated the effects of salinity stress on the plant by balancing the osmotic potential.In conclusion,NO3−-N played both nutritional and osmotic roles in xa Willd.in saline conditions.Keywords Tamarix laxa willd.Salt stress.NO3−-N. Osmoregulation.ProlineIntroductionSalinity reduces plant growth through osmotic stress, ion toxicity,and consequently nutritional stress (Nublat et al.2001).Many studies showed that improving of the nutritional status of plants by nitrogen or phosphorus fertilizers may mitigate the negative impacts of increased salinity and promote thePlant Soil(2010)331:57–67DOI10.1007/s11104-009-0231-7Responsible Editor:John McPherson Cheeseman.X.Ding:S.Zhang:F.Zhang:G.Mi:G.Feng(*) College of Resource and Environmental Science, China Agricultural University;Key Laboratory of Plant Nutrition,MOA;Key Laboratory of Plant-Soil Interactions,MOE, Beijing100193,People’s Republic of Chinae-mail:fenggu@C.TianXinjiang Institute Ecology and Geography,Chinese Academy of Sciences,Urumqi830011,People’s Republic of ChinaJ.SongCollege of Life Science,Shandong Normal University, Jinan250014,People’s Republic of Chinagrowth of plants(Ebert et al.2002;Papadopoulos and Rendig1983).Such a role is believed as an indirect effect of nitrogen on salinity resistance in plants.Nitrogen(N)shortage is one of the main factors limiting plant growth in many ecosystems,particular-ly in saline soils(Albassam2001;Botella et al.1997). Some saline soils,however, e.g.nitric saline soil (belonging to the Typic Salorthids)in Turpan Basin, Xinjiang,northwestern China,contain nitrate concen-tration as high as2–16g kg−1in0–30cm soil layer (Wang et al.1993;Yang1999).Several plant species grow in these nitric saline soils,such as Tamarix spp., Phragmites australis L.,Suaeda pterantha(Kar.et Kir.)Beg,Kalidium foliatum(Pall.)Moq,Halopeplis pygmaea(Pall.)Bge,Saliconia europaea L., Halostachys caspica(Bieb.)C.A.Mey.,Artemisia anethifolia Web.ex Stechm,Aeluropus pungens(M. Bieb.)C.Koch.,and Karelinia caspica(Pall.)Less (Xi et al.2006).Previous research on the relationship between N and salt resistance in plants has mainly focused on the assimilation of N and/or the secondary metabolism of N-containing compounds.For example,increased N supply can promote salt resistance in plants by accumulation of soluble N containing organic com-pounds(e.g.,proline,glycinebetaine and free amino acids)under salinity stress(Dubey and Pessarakli 1995).The possible role of these compatible solutes is in protecting the plants against photoinhibition (Hayashi et al.1997;Holmström et al.2000;Yang et al.2007),reactive oxygen species(Demiral and Türkan2004;Heidari and Mesri2008)or osmotic stress in plants growing under salt stress(Hasegawa et al.2000;Shen et al.1999;Yancey2005).Salinity decreases NO3−uptake by roots(Martinez and Cerda1989),inhibits the activity of nitrate reductase(Campbell1988),and reduces NO3−trans-location from roots to shoots(Gouia et al.1994).On the other hand,salinity stress tends to result in an increase accumulation of proline(Moghaieb et al. 2004;Parida and Das2005)in non-halophytic plants (Albassam2001)and also in halophytes(Khan et al. 2000),and the levels of nitrogenous solutes rises as N supply increases,e.g.,Spartina alterniflora(Colmer et al.1996).NO3−is the main form of N uptake by angiosperms (Martinoia et al.1981).When NO3−absorption exceeds reduction capacity,the excess NO3−will be stored in vacuoles(Blom-Zandstra and Lampe1983).Some ecological factors,e.g.light intensities(Blom-Zandstra and Lampe1985)or osmotic stress(Ourry et al.1992),may suppress the activity of nitrate reductase and result in NO3−accumulation in vacuoles.Marschner(1986)had hypothesized that the storage of NO3−in vacuoles might play a role in osmotic regulation when plants were growing in osmotic stress.However,previous studies have mainly focused on the nutritional function of NO3−, just a few studies have been conducted to assess Marschner’s hypothesis by testing the direct contri-bution of NO3−to the salt resistance of plants,and the results varied.For example,Stienstra(1986)showed that NO3−did not have a specific function in osmotic adjustment in Aster tripolium L.,a halophytic plant, grown in a nutrient solution with either a continuous or an intermittent NO3−supply.By contrast,Song et al.(2006a)suggested that NO3−not only has a nutritional role for growth of the euhalophyte Suaeda physophora,but also directly contributes to osmotic adjustment.In order to test Marschner’s hypothesis,a recretohalophyte(a halophyte with salt excretion glands),Tamarix laxa,was evaluated in hydroponic culture under controlled conditions,with three NO3−treatments under low(1mM)and high(300mM) NaCl-salinity.Materials and methodsPlant materialBranches of xa Willd.of0.5±0.1cm in diameter were collected in November2006,from Fukang, Xinjiang,China(44°18′N,87°55′E).Plant samples were chopped into10–12cm segments,dipped in water for24h,sterilized with0.5%potassium permanganate for30min,and then washed with tap water.The basal part of each segment was dipped in 0.1%rooting powder(ABT Rooting Powder®,pro-duced by ABT Research and Development Center of Chinese Academy of Forestry,Beijing,China)for 10h.The segments were then cultured in quartz sand for rooting and supplied initially with tap water. When new twigs appeared,the plants were cultured with1/10strength nutrient solution for30d and then with1/2strength nutrient solution renewed every 2days for another40days.The nutrient solution composition at full strength was:0.05mM Ca(NO3)2,2.95mM CaCl2,1mM K2SO4,2mM MgSO4, 1mM KH2PO4,90μM Fe-EDTA,46μM H3BO3, 9.1μM MnCl2,0.32μM CuSO4,0.76μM ZnSO4, 0.56μM Na2MoO4,1mM NaCl.The pH was adjusted each day to6.5±0.1with KOH or H2SO4. The pots were in a greenhouse with a light intensity of 480μmol m−2s−1,and temperature of30±3°C in day and24±3°C in night.The relative humidity was50–60%.In order to prevent salt accumulation in the quartz sand,relatively large volumes of fresh nutrient solution was used to irrigate the pots to leach out any excess salts every2days.Experimental designPlants were washed with deionized water,then transferred into1/2strength nutrient solution for 7days.The fresh weight of each seedling was measured,and similar-sized seedlings with fresh weight5.0±0.2g were selected before the pretreat-ment.The experiment was arranged in a completely randomized design with two factors:(1)two salinity levels,1mM(control)and300mM NaCl(salinity);(2)three nitrogen levels,0.05,1and10mM NO3−-N supplied as Ca(NO3)2.The extra Ca2+concentration in different treatments was balanced by CaCl2.In order to avoid osmotic shock,300mM NaCl was applied gradually by adding50mM NaCl per day. The plants were cultured in2L porcelain pots.Each pot contained two seedlings.Three seedlings of similar fresh weight per treatment were collected for dry weight(DW)when the final salinity concentra-tions were reached(as the initial dry weight for calculating relative growth rate).There were six replicates for each treatment,in which three replicates were used for evaluating biomass,and the other three were used for determining physiological parameters. The experiment was terminated24days after final salinity concentrations were reached.The twigs and roots were separated,and fresh weight(FW)was recorded.A sub-sample of fresh twigs samples of each plant was frozen in liquid N2.The remaining plant tissue samples were dried in an oven at80°C for 72h and dry weights(DW)were measured.Water content(WC)was calculated as:(FW-DW)/DW.The concentrations of Na+,K+and organic N were measured in oven-dried samples.Osmotic potential, and the concentrations of Cl−,NO3−and proline,were measured in samples frozen in liquid N2.Determination of the relative growth rates of plantsPlants were sampled at the beginning of treatments and at the end of the experiment.Relative growth rate(RGR) was calculated using the equation(Botella et al.1997):RGR¼log e W2Àlog e W1ðÞ=t2Àt1ðÞNote:W1and W2represent plant fresh weight at harvest1(1day after the final salinity concentrations were reached)and harvest2(at the end of the experiment)respectively,over the harvest interval t1 to t2(1to24days).Determination of inorganic ions,proline,amino acids, and organic N in plantsFrozen plant material(vegetative branches and roots of xa)was extracted with boiling distilled water, and Cl−and NO3−were determined after the solution was filtered.NO3−was determined by the colorimetric method(Cataldo et al.1975)(UV-120-02Spectro-photometer,Shimadzu,Kyoto,Japan),and Cl−was determined by0.03mM AgNO3titration method, with5%K2CrO4as indicator.Frozen plant tissue was also ground in10%acetic acid,and the ninhydrin colorimetric method was used for the determination of amino acids(Moore and Stein1954),or the concentra-tion of proline(Troll and Lindsley1955).For cation determinations,about15-mg dry sample was put in a muffle stove to be ashed.The ash was dissolved in 0.1ml of concentrated nitric acid and then diluted to a volume of20ml with deionized water.The concen-trations of Na+and K+were determined by flame photometry(Model2655-00Digital Flame Analyzer, Cole-Parmer Instrument Company,Chicago,USA). Dry plant samples were ground,and analyzed for organic N using the Kjeldahl method(Shi1994).Determination of osmotic potential(Ψs)The frozen plant tissues were put into a syringe to thaw. The liquid squeezed from the plant tissues was analysed using a freezing point osmometer(Fiske210;Advanced Instruments Inc.,Norwood,MA,USA)to measure the ic value(the value reading from the instrument).The tissue osmotic potential of solutes was calculated as Ψs=–ic RT,where i is ionization constant of the solute, c is the molar concentration of the solute,R is theuniversal gas constant and T is the temperature in degrees Kelvin(Song et al.2006a).Vacuoles account for most of the volume of the twigs, particularly in recretohalophyte plants which have large water storage cells(Bosabalidis and Thomson1985; Thomson1975).For the present analyses,the volume of the cytoplasm is assumed to occupy10%of cells. Therefore we calculated the average concentration of each inorganic ion in vacuole or organic solute in cytoplasm by the content of each individual osmolyte and the tissue water content with the volume ratio of vacuole vs cytoplasm9:1.The osmotic potential(Ψ)of each individual osmolyte,such as Na+,K+,Cl−,NO3−, or proline,was calculated byΨ=-nRT/V,where n is the number of solute molecules,R is the universal gas constant,T is temperature in°K,and V is the volume in liter.Osmotic coefficients of the solutes in tissue water were assumed to equal1(Song et al.2006a).The estimated contributions of each individual solute(C es)to the tissue osmotic potential was calculated using the equation:C es(%)=Ψ/Ψs×100.Statistical analysisAll data were subjected to a two-way ANOVA using the SAS™software(SAS Institute Inc.1989). Treatment means were compared by least significant differences(LSD)at P=0.05.ResultsEffect of NO3−-N on the growth of xaunder salinity stressNo differences were observed in the growth of xa twigs growing at1and10mM NO3−-N at either NaCl level(P<0.05)(Table1).However,twig fresh weights were much lower when grown at 0.05mM NO3−-N under either NaCl level(Table1). There were no significant differences in root fresh weights among the three levels of NO3−-N under either NaCl levels(Table1).Root/twig ratios were significantly higher in plants grown at 0.05mM NO3−-N,compared to values for plants grown at1and10mM NO3−-N under either NaCl levels.These results indicated that increased NO3−supply improved twig growth to some degree regard-less of salinity level.The average twig growth of plants at300mM NaCl was reduced compared to the low salt treatment,at all NO3−-N levels(Table1).Twig growth at 0.05mM NO3was similar at both1and300NaCl but differed between the two salt levels at1and 10mM nitrate.Twig fresh weight under1mM NaCl at 1and10mM NO3−-N was121%and133%higher respectively compared to values obtained at 300mM NaCl,whereas average root fresh weightTable1Effects of NaCl and NO3−-N on the growth of root and twig,root/twig ratio and relative growth rate of xa seedlings. Plants were treated with1or300mM NaCl and0.05,1or10mM NO3−-N for24daysNaCl(mM)NO3−-N(mM)increments of f.wt(g/plant)Root/Twig RGR(g·g−1·day−1)Twig Root1mM0.05 3.90b a 1.39a0.36a0.083b111.64a 2.16a0.19b0.111a1013.07a 2.49a0.19b0.103aMean b9.54A 2.01A0.24B0.097A300mM0.05 3.08b 1.08a0.35a0.051b1 5.27a 1.60a0.30b0.094a10 5.60a 1.58a0.28b0.083aMean 4.65B 1.75A0.31A0.080BAll data are means of3replicationsIncrements of f.wt(g/plant)were calculated by fresh weight at the end of the experiment minus fresh weight at the beginning of treatments a Values marked with different letter represented significant difference at P=0.05level across all NO3−-N levels at a given NaCl level b Means value for each NaCl level.Means values marked with different capital letter indicate significant differences at P=0.05level between NaCl levelsunder 300mM NaCl did not differ from that at 1mM NaCl (Table 1).Compared with 1mM NaCl,the average root/twig ratio was increased under the 300mM NaCl treatment,but the average relative growth rate (RGR)decreased (Table 1).These results indicated that 1mM NO 3−-N was enough to support maximal twig growth of xa under high salinity.The concentrations of NO 3−-N,Cl −,Na +,K +in twigs and roots of xaNO 3−concentrations in twigs of xa were enhanced with increasing NO 3−-N supply under 1or 300mM NaCl (Fig.1a ).Similar trends of NO 3−concentrations were found in roots (Fig.1b ).Compared with plants growing at 1mM NaCl,300mM NaCl reduced the NO 3−concentration in twigs of plants growing at both 1mM NO 3−-N and 10mM NO 3−-N (P <0.05),but did not affect the NO 3−concentration in roots (Fig.1a,b ).The concentration of Cl −in the twigs decreased with increasing NO 3−-N under both NaCl level except at 10mM NO 3−-N and 300mM NaCl treatment where Cl −concentration showed no significant difference comparing to 1mM NO 3−-N and 300mM NaCl treatment (Fig.1c ).In roots,the concentrations of Cl −were significantly higher at 300mM NaCl than those in 1mM NaCl at all NO 3−-N levels (Fig.1d ).NaCl concentration (mM)NaCl concentration (mM)(m o l L -1 w a t e r i n t h e v a c u o l e )(m o l L -1 w a t e r i n t h e v a c u o l e )0.00.10.20.30.40.50.00.10.20.30.40.5abc10 mmol·L -1NO 3--N1 mmol·L -1NO 3--N 0.05 mmol·L -1NO 3--NFig.1NO 3−(a ,b )and Cl −(c ,d )concentrations in the vacuole in twigs and in roots of xa ,which were treated with 1or 300mM NaCl and 0.05,1or 10mM NO 3−-N for 24daysComparing to low NO 3−-N supply,medium and high NO 3−-N levels reduced Cl −concentration in root at 300mM NaCl,while no significant differences were observed between the three NO 3−-N levels at 1mM NaCl (Fig.1d ).These results implied that higher NO 3−-N supply reduced Cl −uptake.Salinity increased the concentration of Na +in both twigs and roots (Fig.2a,b ).At 1mM NaCl,the concentration of Na +in twigs and in roots did not differ with increasing NO 3−-N supply.At 300mM NaCl,the Na +concentration was higher at 0.05mM NO 3−-N than at 1or 10mM NO 3−-N (Fig.2a,b ).These results indicated that supply of higher NO 3−-N levels might have reduced theconcentrations of Na +in both twigs and roots under higher salinity.K +concentration both in twigs and in roots were not significantly different among NO 3−-N treatments under either NaCl level (Fig.2c,d ).Contents of total N,organic N,and NO 3−-N in twigs and roots of xa with increasing supply of NO 3−-N at 1and 300mM NaClThe contents of total N (N total ),organic N (N org )and NO 3−-N in both twigs and roots were all enhanced with increasing supply of NO 3−-N under both NaCl levels compared to the values at 0.05mM NO 3−-N(m o l L -1 w a t e r i n t h e v a c u o l e )0.00.10.20.30.40.51300NaCl concentration (mM)(m o l L -1 w a t e r i n t h e v a c u o l e )1300NaCl concentration (mM)ac10 mmol·L -1NO 3--N1 mmol·L -1NO 3--N0.05 mmol·L -1NO 3--NFig.2Effect of NO 3−-N supply on the concentrations of Na +(a ,b ),K +(c ,d )in the vacuole in twigs and roots of xa which were treated with 1or 300mM NaCl,with 0.05,1or 10mM NO 3−-N for 24dayssupply(Table2).In the high salinity treatment (300mN NaCl),the contents of either total N(N total), or organic N(N org)in twigs were similar between treatments of1and10mM NO3−-N,whereas NO3−-N contents in twigs were significantly different across all three NO3−-N levels at this salinityThe concentrations of total N(N total)and organic N in twigs were enhanced in the300mM NaCl treat-ment compared with those at1mM NaCl,whereas NO3−concentrations were reduced(P<0.05).Salinity had no significant effect on the concentrations of total N(N total)and NO3−-N in roots.Effect of NO3−-N supply on the osmotic potential (Ψs)and the estimated contribution(C es)of Na+, K+,Cl−and NO3−toΨs in twigs of xaThe NO3−-N supply had no effect on theΨs in twigs of xa,while salinity significantly reduced theΨs (Table3).The estimated contribution of Na+toΨs (C Na)decreased with increasing NO3−-N supply at 300mM NaCl(Table3).However,there were no significant differences in the C Na among the three NO3−-N levels at1mM NaCl.C Na increased with salinity,from about7.23%at1mM NaCl up to 35.78%at300mM NaCl(Table3).The estimated contribution of Cl−toΨs(C Cl) declined with the increasing NO3−-N at both NaCl level.The decrease in contribution of Cl−with increasing NO3−-N was more marked at1mM NaCl than at300mM although the interaction was not statistically significant(Table3).The estimated contribution of K+toΨs(C K) decreased with increasing NO3−-N supply at low salinity,but there were no significant differences among the different NO3−-N levels under300mM NaCl (Table3).C K decreased with increased NaCl supply.The estimated contribution of NO3−toΨs(C NO3−) increased with the increase of NO3−-N at both1mM and300mM NaCl,whereas it was lower at high salinity at any given NO3−level(Table3).Effect of NO3−-N supply on the concentrationand the estimated contribution of proline toΨsin twigs of xaBased on the assumption that proline was restricted to the cytosol,and that that accounted for10%of the total cell volume,there was a significant increase inTable2The effects of NO3−-N on the contents of total N,organic N and NO3−-N in twigs and roots of xa which were treated with1or300mM NaCl and0.05,1or10mM NO3−-N for24daysNaCl NO3−-N Ntotal(mg·g−1DW)Norg(mg·g−1DW)N NO3−(mg·g−1DW)(mM)(mM)Twig Root Twig Root Twig Root10.0520.88c d13.60b20.34b12.57b0.54c0.91b137.80b22.87a32.11a17.98a 5.69b 4.37a1043.50a25.94a34.72a20.59a8.79a 3.74aMean e34.06B f20.80A29.06B17.05B 5.01A 3.01A 3000.0526.51b16.26b25.60b15.38c 1.03c0.87b 139.80a25.25a35.42a19.82a 4.9b 5.44a1037.83a22.75a34.09a17.48b 5.34a 5.27aMean34.71A21.42A31.70A17.56A 3.76B 3.86A Analysis of Variance(F Values)Salinity(S)8.84b 1.27NS 6.62a 1.47NS10.96b0.77NS NO3−-N level(N)15.85c78.81c67.05c25.02c15.85c95.09cS×N 3.69NS8.61NS 2.94NS8.59NS8.71b 4.03NSa denotes significant difference at P=0.05,b denotes significant difference at P=0.01,c denotes significant difference at P=0.001,NS denotes no significant difference.Data represent F valuesd Within each column,values with different letter are significantly different at P=0.05level across all NO3−-N levelse Mean value for each NaCl levelf Mean values with different capital letter are significantly different at P=0.05level between NaCl levelsproline concentration at 300mM NaCl compared to the low salinity treatment.Concentrations of proline rose with increasing NO 3−-N supply at 1mM NaCl;However,at 300mM NaCl,concentrations of proline were maximum at 1mM NO 3−-N supply,which indicated that 1mM NO 3−-N supply was enough for structural growth;however,as the level of NO 3−-N supply increased to 10mM the concentration of proline decreased as more NO 3−were being stored in the vacuoles.The concentration of proline was the lowest at 0.05mM NO 3−-N which implied that the proline synthesis was limited because of nitrogen deficiency (Fig.3a ).The estimated contribution of amino acids to Ψs (C pro )increased with increasing NO 3−-N at 1mM NaCl.At 300mM NaCl,it increased significantly when the NO 3−-N supply was increased from 0.05to 1mM,but then dropped at 10mM NO 3−-N (Fig.3b ).In general,the estimated contribution of proline to Ψs (C pro )was considerably lower at both salinities and all three NO 3−-N levels compared with the inorganic solutes described above:the maximum contribution of proline accounted for only 3.6%of Ψs .DiscussionNitrogen is an essential nutrient for higher plants.Salinity may suppress the uptake and assimilation of nitrate in plants,which results in nutritional disorder and growth inhibition (Dluzniewska et al.2007;Marschner 1986).In the present study,high salinity reduced twig growth and the relative growth rate (RGR)of xa plants (Table 1).However,the average total nitrogen or organic nitrogen contents in plants grown under 300mM NaCl were higher than those grown under 1mM NaCl,while the average NO 3−concentration was lower at high NaCl (Table 2).Such results indicated that the uptake and assimilation of NO 3−in xa were not suppressed by high salinity.Increasing the nitrogen supply under either level of NaCl supply improved the RGR of xa (Table 1),which can be partly attributed to the nutritional role of N as many other researchers have concluded (Irshad et al.2008;Leidi et al.1992).In order to lower water potential,halophytes accumulate large amounts of inorganic ions in the vacuole and synthesize a relatively small amount of low molecular weight organic compounds to balanceTable 3The effects of NO 3−-N onthe osmotic potential (Ψs ),water content (WC),the estimated contribution of Na +(CNa +),K +(CK +),Cl −(CCl −)and NO 3−(C NO 3−)to osmotic potential in twigs of xa which were treated with 1or 300mM NaCl and 0.05,1or 10mMNO 3−-N for 24days NaCl NO 3−-N Ψs WCCNa +CCl −CK +C NO 3−(mM)(mM)(MPa)(ml·g −1DW)(%)(%)(%)(%)10.05−1.30a d 3.03c 7.52a 54.2a 20.72a 2.4b 1−1.25a 3.81b 7.85a 28.94b 21.77a 29.2a 10−1.61a 4.14a 6.32a 11.89c 16.47b 29.47a Meane−1.39A f3.66A 7.23B 24.00B 19.46A 15.44A 3000.05−1.71a 3.87a 42.16a 46.61a 12.57a 2.29c 1−1.48a 3.96a 34.03b 27.4b 15.99a 10.72b 10−1.55a 3.90a 31.14b 27.08b 14.12a 19.93a Mean−1.58B 3.91A 35.78A 33.7A 14.22B 10.31B Analysis of Variance (F Values)Salinity (S)0.16NS 10.67a 83.77c 56.07c 41.03c 35.53b NO 3−-N level (N)0.68NS 17.2c 1.37NS 4.20a 6.08a 61.01c S ×N0.03NS 14.51b0.97NS0.7NS3.96NS10.04cadenotes significant difference at P =0.05,b denotes significant difference at P =0.01,c denotes significant difference at P =0.001,NS denotes not significant difference.Data represent F valuesd Within each column,values with different letter are significantly different at P =-0.05level across all NO 3−-N levels e Mean value for each NaCl levelfMean values with different capital letter are significantly different at P =0.05level between NaCl levels3−on the osmotic potential (Ψs ),water content (WC),the estimated contribution of Na +(CNa +),K +(CK +),Cl −(CCl −)and NO 3−(C NO 3−)to osmotic potential in twigs of xa which were treated with 1or 300mM NaCl and 0.05,1or 10mM NO 3−-N for 24daysthe osmotic pressure in the cytoplasm (Hasegawa et al.2000;Zhao et al.2003).Song et al.(2006b )demonstrated that euhalophyte Suaeda physophora is able to compartmentalize inorganic ions,especially Na +in the vacuole,and synthesize a relatively small amount of organic solutes to balance the osmotic pressure in the cytoplasm.In the present study,xa accumulated Na +and Cl −in twigs under high salinity (Figs.1,2),which would lower osmotic potential in the twigs (Table 3).However,it is difficult to directly test the real concentration of the solutes:it was as-sumed that inorganic solutes were mainly distributed in vacuole and that organic solutes entirely accumu-lated in cytoplasm.In the present study,we estimated the concentrations of inorganic or organic solutes by tissue water volume and the general ratio of vacuole to cytoplasm.The distribution was based on the assump-tion,justified by anatomical studies,that the vacuole accounted for 90%and the cytoplasm and organelles for 10%of the overall cell volume (Di Martino et al.2003).Therefore the relative estimated-contribution of the inorganic or organic solutes to osmotic potential can be quantified (Silveira et al.2009).Although Marschner (1986)had hypothesized that NO 3−stored in vacuoles might play a role in osmotic regulation when plants were growing in osmotic stress,whether or not NO 3−plays this role and directly contributes to salt resistance of plants is still poorly understood.As noted in the introduction,the answer may depend on the species being considered.Our present study showed that NO 3−-N supply significantly enhanced the contribution of NO 3−to osmotic potential,from 2%to 20%in twigs of xa under high NaCl (Table 3).Such result suggested that in addition to the nutritional role,NO 3−accumulation in the vacuoles could play an important role in balancing the osmotic potential in xa under high salinity with adequate NO 3−-N supply.The interaction between Cl −and NO 3−may strongly affect the contribution of both anions to osmotic regulation in plant.The use of more NO 3−but less Cl −ions for osmotic adjustment may prevent Cl −toxicity in Suaeda physophora (Song et al.2006a ).Cl −present in the expanded leaves of certain species is associated with chlorosis and death,and these injuries occur even when the Na +concentration is low in the leaves (Greenway and Munns 1983).However,in our present study,more NO 3−but less Cl −or Na +might be accumulated in the vacuole for osmotic adjustment at higher NO 3−supply,compared with 1mM NO 3−.As NO 3−supply increased,the decrease in the estimated contribution of Cl −to osmotic potential in xa was compensated by an increase in that of NO 3−(Table 3).This can be attributed to competition between NO 3−and Cl −for transport systems,which are proposed to play significant roles in uptake or the xylem loading of NO 3−and Cl −(Cerezo et al.1997;Köhler and Raschke 2000).Twigsc cbaa b5101520253013001300NaCl concentration (mM)NaCl concentration (mM)P r o l i n e c o n c e n t r a t i o n(m m o l L -1w a t e r i n t h e c y t o p l a s m )0.01.02.03.04.05.0P e r c e n t (%)ab10 mmol·L -1NO 3--N1 mmol·L -1NO 3--N0.05 mmol·L -1NO 3--NFig.3Proline concentration (a )and the estimated contribution of proline to Ψs (b )in the cytoplasm in twigs of xa which were treated with 1or 300mM NaCl and 0.05,1or 10mM NO 3−-N for 24days。
Wu et al.,2015-Revised Ti-in-biotite geothermometer for ilmenite- or rutile-bearing
Article Earth ScienceRevised Ti-in-biotite geothermometer for ilmenite-or rutile-bearing crustal metapelitesChun-Ming Wu •Hong-Xu ChenReceived:1July 2014/Accepted:19September 2014/Published online:19December 2014ÓScience China Press and Springer-Verlag Berlin Heidelberg 2014Abstract In the present study,the Ti-in-biotite geother-mometer was revised using more than 300natural rutile-or ilmenite-bearing metapelites collected worldwide.The formulation was empirically calibrated as ln ½T ð C Þ ¼6:313þ0:224ln ðX Ti ÞÀ0:288ln ðX Fe ÞÀ0:449ln ðX Mg Þþ0:15P ðGPa Þ,with X j ¼j =ðFe þMg þAl VI þTi Þin biotite,assuming ferric iron content of 11.6mol%of the total iron in biotite.This thermometer is consistent with the well-calibrated garnet–biotite thermometer within error of ±50°C for most of the calibrant samples and can successfully distin-guish systematic temperature changes of different meta-morphic zones in both prograde and inverted metamorphic terranes as well as thermal contact aureoles.Thus,the thermometer truthfully reflects real geologic conditions and can be applied to TiO 2-saturated metapelites metamor-phosed at the crustal level within the calibration ranges (450–840°C,0.1–1.9GPa,X Ti =0.02–0.14in biotite).Keywords Ti content ÁBiotite ÁCalibration ÁGeothermometer ÁApplication ÁError1IntroductionThe development of valid geothermobarometers has been a crucial component of petrologic research for more than80years.Metapelites,in particular,are quite sensitive to metamorphic pressure–temperature (P –T )conditions,and metapelitic mineral assemblages typically record the tectono-metamorphic evolution of metamorphic terranes.Therefore,thermobarometers applicable to metapelites are powerful tools in retrieving information about metamorphic processes.Such geothermobarometers include the valid garnet–biotite thermometer [1],the garnet–Al 2SiO 5–plagioclase–quartz (GASP)barometer [2],the garnet–biotite–plagioclase–quartz (GBPQ)barometer [3],the garnet–muscovite–plagioclase–quartz (GMPQ)thermobarometers [4],and the garnet–bio-tite–muscovite–Al 2SiO 5–quartz (GBMAQ)barometer [5].In fact,constructing concise and easily used monomi-neralogic thermobarometers is a priority for petrologists,which has led to development of the hornblende thermoba-rometer [6,7],the Si-in-phengite geobarometer [8,9],and the Ti-in-biotite geothermometer [10,11].The Ti-in-biotite geothermometer is extremely useful in deciphering the metamorphic temperatures of metapelites,especially when garnet is absent in TiO 2-saturated rocks.However,further work is required for this thermometer,particularly owing to the limited ranges of calibration P –T conditions and chem-ical compositions of biotites.In the present study,the Ti-in-biotite thermometer was revised based on abundant me-tapelitic samples collected worldwide;then,the applicability of the thermometer was tested by applying it to prograde and inverted metamorphic terranes and thermal contact aureoles.2Calibration of the Ti-in-biotite geothermometer 2.1Calibration data setIn total,334rutile-and/or ilmenite-bearing natural me-tapelitic samples collected worldwide were collated basedElectronic supplementary material The online version of this article (doi:10.1007/s11434-014-0674-y )contains supplementary material,which is available to authorized users.C.-M.Wu (&)ÁH.-X.ChenCollege of Earth Science,University of Chinese Academy of Sciences,Beijing 100049,China e-mail:wucm@Sci.Bull.(2015)60(1):116– DOI 10.1007/s11434-014-0674-y /scpon the existing literature.Of these,24samples (7%of the total data)were discarded owing to possible disequilib-rium;the remaining 310samples were used in the cali-bration.These data are given in Table S1.For graphite-or ilmenite-bearing metapelites,the ferric contents of garnet and biotite were estimated to be 3%and 11.6%,respec-tively,of the total iron [1].These rocks were metamor-phosed under P –T conditions of 450–840°C and 0.1–1.9GPa,respectively,determined simultaneously by applying the garnet–biotite geothermometer [1]and the GASP geobarometer [2],as suggested by Wu and Cheng [12].The chemical ranges are X Fe =0.19–0.55,X Mg =0.23–0.67,and X Ti =0.02–0.14for biotites,on the basis of 11oxygen per formula unit,in which X j ¼j =ðFe þMg þAl VI þTi Þin biotite.It should be stated that such compositional ranges cover more than 98%of the natural biotites in rutile-or ilmenite-bearing metapelites.2.2Calibration of the thermometerHenry and Guidotti [10]and Henry et al.[11]found that there exists a curved Ti-saturation surface in the T –Ti–Mg#space and fitted T ,Ti atoms,and Mg#½¼Mg =ðMg þFe Þ of biotites to an exponential equation,as the Ti-in-biotite thermometer.The empirical formulations (Eqs.1and 2)of their thermometers are described as ln ðTi Þ¼À2:3353þ4:343Â10À9½T ð C Þ 3À1:6718ðMg#Þ3;ð1Þandln ðTi Þ¼À2:3594þ4:6482Â10À9½T ð C Þ 3À1:7283ðMg#Þ3;ð2Þrespectively,on the 22-O basis of biotite.These previous studies demonstrated that the precision of the thermometer is around ±25°C.However,their calibration data were metapelitic samples collected from western Maine and metamorphosed under pressure condi-tions of 0.33GPa [10]and collected from both western Maine and south-central Massachusetts [11]and meta-morphosed under pressure conditions of 0.4and 0.6GPa,respectively,at almost constant pressure conditions.We found that another type of regression model can better describe the temperature dependence of the Ti con-tents of biotites;thus,the Ti-in-biotite geothermometer (Eq.3)was empirically calibrated through multiple regression analysis as follows:ln T C ðÞ½ ¼6:313Æ0:078ðÞþ0:224Æ0:006ðÞln X Ti ðÞÀ0:288Æ0:041ðÞln X Fe ðÞÀ0:449Æ0:039ðÞÂln X Mg ÀÁþ0:15Æ0:01ðÞP GPa ðÞ;ð3Þwith a multiple correlation coefficient of R =0.906obtained when fitting the calibrant data set listed in Table S1.It should be noted that the present formulation is only empirical and lacks strict thermodynamic and crystallo-graphic bases;however,it is valid and practical in the applications described hereafter.2.3Error analysisThe present Ti-in-biotite thermometer (Eq.3)yields similar temperatures to the garnet–biotite thermometer [1]within error of ±50°C for 72%of the total 310calibrant samples (Fig.1a).In contrast,the Ti-in-biotite thermometer of Henry et al.[11]typically overestimates temperatures (Fig.1b)compared to the garnet–biotite thermometer [1].The garnet–biotite thermometer [1]is the most precise and accurate of all thermometers used in metapelites [12].Therefore,we evaluated the accuracy of the present Ti-in-biotite thermometer by comparing the temperature differ-ences between these two thermometers and defined this as the random error of the Ti-in-biotite thermometer.Our results show that the random error of this thermometer is nearly independent of both temperature (Fig.1c)and pressure (Fig.1d)or the chemical compositions of biotites (Fig.1e–g),suggesting that the random error of the new Ti-in-biotite thermometer is evenly distributed,without any bias.Based on the calibrant data set (Table S1),the following statements can be made:(a)an error of ±0.2GPa of input pressure translates to a temperature error of ±14–25°C and (b)analytical error of ±2%for the Ti,Fe,and Mg contents in biotites may translate to temperature errors of ±2–4°C,±1–3°C,and ±1–5°C,respectively.Thus,the random error of this new thermometer was arbitrarily estimated to be ca.±65°C,when considering both for reproduction of calibration temperatures and for applica-tions to natural metamorphic terranes.The absolute error of the present Ti-in-biotite thermometer could not be evalu-ated owing to a shortage of experimental data.3Applications of the Ti-in-biotite geothermometer A valid thermometer should be able to accurately distin-guish systematic temperature changes of different zones in prograde or inverted metamorphic terranes and in thermal contact aureoles,as suggested by Wu and Cheng [12]when evaluating the validity of the garnet–biotite thermometers.The present Ti-in-biotite thermometer has been applied to such metamorphic regions worldwide (Table S2),and it has been demonstrated that this thermometer both reflectssystematic temperature changes and yields similar(but some slightly lower)temperature estimates(Fig.2)to the garnet–biotite geothermometer[1].It appears that the present Ti-in-biotite thermometer (Eq.3)is superior to that of Henry et al.[11];in particular, the latter cannot reflect temperature increases between some neighboring metamorphic zones(Fig.2a,c–e,h).It should be noted that these two Ti-in-biotite thermometers have erroneously indicated temperature decrease from the higher kyanite–biotite zone to the sillimanite zone of the inverted metamorphic terrane in the western metamorphic belt,Juneau,Alaska[17],and from zone7to zone8of the regional thermal contact aureoles of west-central Maine [20].However,the causes of these errors remain unknown. 4DiscussionThe temperature dependence of the Ti contents of me-tapelitic biotites has long been recognized,although the reasons for this dependence are relatively complex.Henry and Guidotti[10]and Henry et al.[11]argued that the coupled cation–cation exchanges in biotites,including 2VI R2þ¼VI TiþVI h,2VI Al¼VI TiþVI R2þ,VI R2þþ2IV Si¼VI Tiþ2IV Al,and VI R2þþ2OHÀ¼VI Tiþ2O2À, may be the mechanisms by which Ti atoms are incorpo-rated into biotite solution.However,it has been shown that no one cation exchange reaction is solely responsible for the temperature effect illustrated by Fig.8of Henry et al.[11],suggesting that the Ti-in-biotite thermometer merits further study.In their derivation,Henry et al.[11]used graphitic, aluminous,biotite-bearing metapelitic samples from wes-tern Maine and south-central Massachusetts,with input temperatures determined based on locations of isograd reactions and dehydration melting reactions calibrated against the petrogenetic grid of Spear et al.[21]at pres-sures of0.4and0.6GPa,respectively.Although Henry and Guidotti[10]and Henry et al.[11]acknowledged that Ti contents of biotites are negatively related to pressure,their formulations contained no pressure item owing to the narrow pressure range of their calibrant samples.On the contrary,we found that the Ti contents of biotites are positively related to pressure,and pressure is included in the present formulation.Another possible reason for the superiority of the new Ti-in-biotite thermometer is the splitting of the Mg#½=Mg/(MgþFe)]item into two items,i.e.,X Fe and X Mg,in our regression analysis.However,a strict theoretical basis for the superiority remains elusive at present.Accordingly, it should be noted that the new formulation is merely an empirical equation,although it deserves further calibration in the future and can be applied already in certain situations.5ConclusionsThe calibration P–T ranges(450–840°C,0.1–1.9GPa)of the Ti-in-biotite geothermometer were broadened tofit greenschist to granulite facies,TiO2-saturated,rutile-and/ or ilmenite-bearing metapelites.This thermometer not only yields similar temperature estimates to the well-calibrated garnet–biotite thermometer,but also clearly distinguishes systematic temperature changes in different zones of pro-grade or inverted metamorphic regions and thermal contact aureoles.The random error of this thermometer has been shown to be no more than approximately±65°C. Acknowledgements This work was supported by the National Natural Science Foundation of China(41225007).Conflict of interest The authors declare that they have no conflict of interest.References1.Holdaway MJ(2000)Application of new experimental and garnetMargules data to the garnet-biotite geothermometer.Am Mineral 85:881–8922.Holdaway MJ(2001)Recalibration of the GASP geobarometer inlight of recent garnet and plagioclase activity models and ver-sions of the garnet-biotite geothermometer.Am Mineral 86:1117–11293.Wu CM,Zhang J,Ren LD(2004)Empirical garnet-biotite-pla-gioclase-quartz(GBPQ)geobarometry in medium-to high-grade metapelites.J Petrol45:1907–19214.Wu CM,Zhao GC(2006)Recalibration of the garnet-muscovite(GM)geothermometer and the garnet-muscovite-plagioclase-quartz(GMPQ)geobarometer for metapelitic assemblages.J Petrol47:2357–23685.Wu CM,Zhao GC(2007)The metapelitic garnet-biotite-mus-covite-aluminosilicate-quartz(GBMAQ)geobarometer.Lithos 97:365–3726.Gerya TV,Perchuk LL,Triboulet C et al(1997)Petrology of theTumanshet zonal metamorphic complex,eastern Sayan.Petrol 5:503–5337.Zenk M,Schulz B(2004)Zoned Ca-amphiboles and related P-T evolution in metabasites from the classical Barrovian meta-morphic zones in Scotland.Mineral Mag68:769–7868.Massonne H-J,Schreyer W(1987)Phengite geobarometry basedon the limiting assemblage with K-feldspar,phlogopite,and quartz.Contrib Mineral Petrol96:212–2249.Massonne H-J,Schreyer W(1989)Stabilityfield of the high-pressure assemblage talc?phengite and two new phengite barometers.Eur J Mineral1:391–41010.Henry DJ,Guidotti CV(2002)Titanium in biotite from me-tapelitic rocks:temperature effects,crystal-chemical controls, and petrologic applications.Am Mineral87:375–38211.Henry DJ,Guidotti CV,Thomson JA(2005)The Ti-saturationsurface for low-to-medium pressure metapelitic biotites:impli-cations for geothermometry and Ti-substitution mechanisms.Am Mineral90:316–32812.Wu CM,Cheng BH(2006)Valid garnet-biotite(GB)geother-mometry and garnet-aluminum silicate-plagioclase-quartz (GASP)geobarometry in metapelitic rocks.Lithos89:1–23 13.Huang MH,Buick IS,Hou LW(2003)Tectonometamorphicevolution of the eastern Tibet Plateau:evidence from the central Songpan-Garzeˆorogenic belt,western China.J Petrol44: 255–27814.Weller OM,St-Onge MR,Waters DJ et al(2013)QuantifyingBarrovian metamorphism in the Danba structural culmination of eastern Tibet.J Meta Geol31:909–93515.Rı´os C,Garcı´a C,Takasu A(2003)Tectono-metamorphic evo-lution of the Silgara´formation metamorphic rocks in the south-western Santander Massif,Colombian Andes.J S Am Earth Sci 16:133–15416.Ferry JM(1981)Petrology of graphitic sulfide-rich schists fromsouth-central Maine:an example of desulfidation during prograde regional metamorphism.Am Mineral66:908–93017.Himmelberg GR,Brew DA,Ford AB(1991)Development ofinverted metamorphic isograds in the western metamorphic belt, Juneau,Alaska.J Meta Geol9:165–18018.Mezger JE,Chacko T,Erdmer P(2001)Metamorphism at a latemesozoic accretionary margin:a study from the Coastal Belt of the North American Cordillera.J Meta Geol19:121–13719.Novak JM,Holdaway MJ(1981)Metamorphic petrology,min-eral equilibria,and polymetamorphism in the Augusta quadran-gle,south-central Maine.Am Mineral66:51–6920.Holdaway MJ,Dutrow BL,Hinton RW(1988)Devonian andCarboniferous metamorphism in west-central maine:the musco-vite-almandine geobarometer and the staurolite problem revis-ited.Am Mineral73:20–4721.Spear FS,Kohn MJ,Cheney JT(1999)P-T paths from anatecticpelites.Contrib Mineral Petrol134:17–32。
