Respiratory Syncytial Virus Matrix Protein Induces Lung
呼吸道病毒五项临床意义
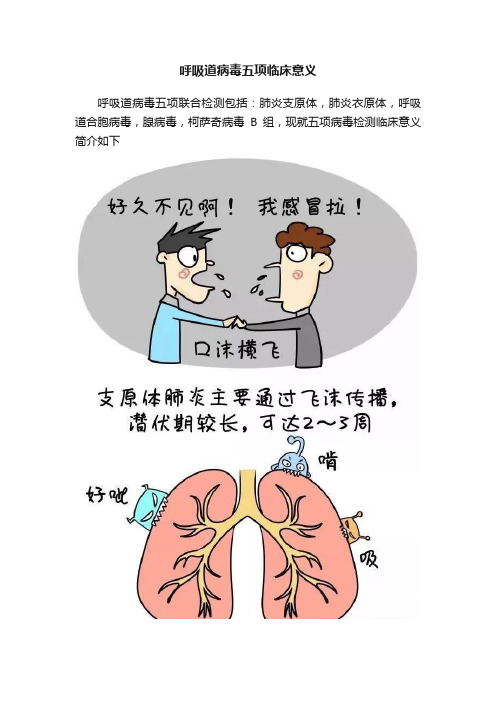
呼吸道病毒五项临床意义呼吸道病毒五项联合检测包括:肺炎支原体,肺炎衣原体,呼吸道合胞病毒,腺病毒,柯萨奇病毒B组,现就五项病毒检测临床意义简介如下1、肺炎支原体肺炎支原体(Mycoplasma pneumoniae,MP)是引起上呼吸道感染、气管支气管炎及非典型肺炎的常见病原体之一。
MP感染可在任何年龄发生,尤其以5~20岁更多见。
MP通过飞沫,以气溶胶微粒的形式传播,感染后引起肺炎支原体肺炎(Mycoplasmal pneumonia,MPP),每隔3~5年出现1次地区性流行,占各类肺炎总数的10%~20%,入伍新兵患肺炎者30%~50%由MP引起,约占非细菌性肺炎的1/3以上。
由于肺炎支原体感染所致的社区获得性肺炎患者临床表现不典型,经过10-20d的潜伏期,可出现一些非特异性症状如头痛和发热,常伴有无力和干咳,根据临床症状不易作出鉴别诊断,常被忽视而导致严重的合并症。
由于肺炎支原体无细胞膜,作用于细胞膜的抗菌药物对其无杀伤作用,加上其感染初期临床表现不明显,另外,MP感染还可以造成肺外各系统改变,且有死亡病例的报道,已引起临床关注。
因此,及时、有效的进行肺炎支原体感染的实验室诊断变得尤为重要。
肺炎支原体可在呼吸道粘膜上皮内潜伏,部分患者无明显症状;但大部分患者为显性感染。
在3岁以下儿童以上呼吸道感染多见,成人以肺炎表现为主。
肺炎支原体肺炎潜伏期14~21天,起病缓慢,IgM抗体一般在感染后1周出现,3~4周达高峰,可持续存在3~6个月。
2、肺炎衣原体肺炎衣原体(Chlamydia pneumoniae,C.pn)又称TWAR,能引起呼吸道感染,包括肺炎、支气管炎、鼻窦炎、耳炎、咽炎及喉炎等,也与其它疾病如冠状动脉粥样硬化、心肌炎、心内膜炎、急性心肌梗死、恶性肿瘤、脑血管病、肾功能不全、帕金森氏病等有关,因而受到愈来愈多的重视。
C.pn感染是世界各地广泛存在的常见病,一年四季均可发生,无显著的性别差异和地区差异。
呼吸道合胞病毒如何治疗

呼吸道合胞病毒如何治疗引言呼吸道合胞病毒(Respiratory syncytial virus,RSV)是一种常见的引起呼吸道感染的病毒。
RSV主要感染婴儿和幼儿,但也可影响成人。
本文将介绍呼吸道合胞病毒的治疗方法,包括支持性治疗、药物治疗和预防措施。
支持性治疗支持性治疗是呼吸道合胞病毒感染的基本治疗方法,旨在缓解症状,提供舒适和康复环境。
1.保持水分摄入:饮水可缓解喉咙痛和咳嗽,并防止脱水。
2.休息:充足的休息有助于恢复体力和增强免疫力。
3.避免二手烟:二手烟会刺激呼吸道,加重症状。
4.使用加湿器:加湿器可以增加空气湿度,缓解咳嗽和呼吸困难。
药物治疗在呼吸道合胞病毒感染中,药物治疗主要包括抗病毒药物和支持性药物。
抗病毒药物抗病毒药物对呼吸道合胞病毒的治疗作用较有限,主要用于严重感染的高危人群,如早产儿、免疫系统受损者和心肺疾病患者。
常用的抗病毒药物包括:1.瑞磷酸酯:它是一种核苷酸类似物,可抑制病毒复制和传播。
但使用该药物需要医生的指导和监控。
2.进口人免疫球蛋白:通过提供抗体来帮助患者抵抗病毒感染。
这种药物通常用于高危人群。
支持性药物支持性药物主要用于缓解症状和改善患者的舒适度。
1.解热镇痛药:如对乙酰氨基酚可以缓解发热和不适感。
2.去痰药:如氨溴索可以帮助稀化和排出呼吸道分泌物。
3.抗过敏药物:如抗组胺药可缓解过敏症状,如鼻塞和流涕。
4.支气管扩张剂:如沙丁胺醇可以扩张支气管,缓解呼吸困难。
预防措施预防呼吸道合胞病毒感染的最好方法是采取一些预防措施,尤其是对于高危人群。
1.注意个人卫生:勤洗手、避免与感染者亲密接触、避免触摸眼睛、鼻子和口腔等部位。
2.空气消毒:经常通风,定期清洁和消毒房间、公共场所。
3.强化免疫系统:保持良好的营养,适当锻炼,避免疲劳和压力。
4.接种疫苗:对于高危人群,应接种呼吸道合胞病毒疫苗,以减少感染和严重病例的发生。
结论呼吸道合胞病毒是一种常见的呼吸道感染病毒,主要感染婴儿和幼儿。
呼吸道合胞病毒有什么症状和表现

呼吸道合胞病毒有什么症状和表现
呼吸道合胞病毒(Respiratory Syncytial Virus,RSV)是一种主要引起婴幼儿
呼吸道感染的病毒,常见于冬春季节。
RSV感染是导致免疫力薄弱的人群发生急
性下呼吸道感染的主要原因之一。
那么,呼吸道合胞病毒感染都有哪些症状和表现呢?下面将一一为您介绍。
呼吸道合胞病毒感染的常见症状:
1.流感样症状:RSV感染的早期症状类似感冒或流感,患者可能出现
发热、流涕、喉咙痛、咳嗽等症状。
2.呼吸道症状:该病毒主要侵袭呼吸道,导致患者出现呼吸困难、气
促、嘶哑、咳嗽等呼吸困难症状。
3.呼吸道感染:婴幼儿是RSV感染的高危人群,可能呈现气急、反复
呼吸窘迫、喘息等呼吸困难的症状。
4.呼吸道疾病加重:对于原本患有哮喘、慢性支气管炎等呼吸道慢性
疾病的患者,RSV感染可能导致疾病加重,并伴有咳嗽、咳痰、呼吸急促等
症状。
除了上述常见症状外,呼吸道合胞病毒感染还可能引起体温升高、食欲不振、
乏力等全身症状。
需要注意的是,对于免疫力低下的人群以及老年人和婴幼儿,RSV感染可能引发严重并发症,如肺炎等,应及时就医治疗。
综上所述,呼吸道合胞病毒感染的症状多样,早期症状类似于感冒或流感,后
期可能引起呼吸道症状并危及呼吸功能,因此及时发现并治疗RSV感染至关重要。
预防RSV感染的关键在于保持良好的个人卫生习惯,避免接触已经感染的人群,
注重室内通风等措施,有效预防呼吸道合胞病毒感染的发生。
呼吸道合胞病毒病毒感染的病理生理学研究

呼吸道合胞病毒病毒感染的病理生理学研究呼吸道合胞病毒(Respiratory Syncytial Virus,简称RSV)是一种极为常见的呼吸道病毒,它主要通过空气传播途径引起人们的感染。
RSV是小儿的常见病原体,尤其是对于6个月以下的婴儿来说,RSV感染是一种比较严重的疾病。
此外,RSV感染也是老年人及免疫功能低下者的常见疾病。
近年来,随着研究的不断深入,对于RSV感染的病理生理学研究也逐渐受到人们的重视。
RSV病毒感染主要通过呼吸道黏膜直接侵入机体,进入上呼吸道并迅速开始繁殖。
RSV的感染主要分为两种类型,即轻型感染和重型感染。
轻型感染的症状主要是像普通感冒一样,表现为咳嗽、流鼻涕、发热等症状。
但重型感染可能会引起呼吸道的炎症反应,严重时甚至会导致死亡。
RSV感染后会引起机体免疫反应,免疫细胞被捕获、活化并释放出细胞因子和趋化因子,导致呼吸道炎症反应的增强和扩展。
在RSV感染初期,受感染的细胞会释放出炎症因子,如IL-8、TNFα等,从而引起病理性的炎症反应。
炎症反应在RSV感染初期一定程度上对机体有保护性作用,但是如果炎症反应过度,就会引起机体的免疫损伤,导致严重的心肺并发症。
近年来,有关RSV感染的病理生理学研究已经有了很大的进展,其中主要包括以下几个方面:1. RSV与呼吸道细胞的相互作用RSV感染与呼吸道黏膜的柱状上皮细胞紧密相关,RSV病毒会对柱状上皮细胞的基础层细胞和基底细胞进行感染,从而引起上皮细胞损伤和坏死。
此外,RSV 病毒感染后会引起炎症因子的释放,进一步诱导细胞凋亡和细胞受体的表达,从而增强了RSV感染和免疫反应的相互作用。
2. 免疫细胞对RSV感染的参与免疫细胞在RSV感染中也扮演着非常重要的角色。
免疫细胞可有效吞噬病毒并释放细胞因子,从而起到调节机体免疫反应的作用。
在RSV感染中,免疫细胞主要包括巨噬细胞、树突状细胞和自然杀伤细胞,它们可通过吞噬病毒、抑制炎症反应和促进细胞增殖等方式参与RSV感染过程。
医学微生物学基本词汇英汉对照表

单词注释单词注释a cacquired immunodificie ncy syndrome 获得性免疫缺陷综合征(艾滋病)schlamydia psittaci 鹦鹉热衣原体active immunit 自动免疫chlamydia trachomatis 沙眼衣原体acute infection 急性感染chronic infection 慢性感染acyclovir 无环鸟苷classical biotype 古典生物型adenoviridae 腺病毒科clostridium 梭状芽胞杆菌属adenovirus 腺病毒cl.botulinum 肉毒梭菌adhesin 粘附素cl.difficile 艰难梭菌adsorption 吸附cl.perfringens 产气荚膜杆菌amantadine 金刚烷胺cl.tetani 破伤风梭菌amphotericin b 二性霉素b coagulase 血浆凝固酶anaerobicbacteria厌氧菌coccidioides immitis 厌酷球孢子菌antibiotic 抗生素coccus 球菌antigenic drift 抗原性漂移colonizzation factor 定居因子antigenic shift 抗原性转变complex symmetry 复合对称antisepsis 防腐continuous cell culture 传代细胞培养antitoxicimmunity抗毒素性免疫 cord factor 索状因子antitoxin 抗毒素core 核心apparentinfection显性传染coronaviridae 冠状病毒科arenaviridae 沙粒病毒科corynebacterium 棒状杆菌属artificial activeimmunity人工自动免疫 c.diphtheriae 白喉杆菌artificialpassiveimmunity人工被动免疫 coxiella 柯克斯体属aspergillus 曲霉菌coxsackie virus 柯萨奇病毒asepsis 无菌cryptococcus neoformans 新新型隐球菌assembly 装配cubic symmetry 立体对称avp 抗病毒蛋白cytocitic infection 杀细胞感染bacillicalmette-guerin, bcg卡介苗cytomegalovirus 巨细胞病毒bacillus 杆菌cytopathogenic effect 细胞致病作用b.anthraci 炭疽杆菌c-onc 细胞原癌基因b dbacteremia 菌血症defective interfering particles 缺损干扰颗粒bacteriocin 细菌素dengue virus 登革病毒bacteriod 类杆菌dermatophytes 皮肤丝状菌b.fragilis 脆弱类杆菌diphtheria 白喉b.melaninogenicus黑色素类杆菌 diplococcus 双球菌bacteriophage 噬菌体diploid cell culture 二倍体细胞培养bacterium 细菌disinfection 消毒blastomyces dermatitidis 皮炎芽生菌enteroniavasive escherichiacol,eiec肠侵袭性大肠杆菌bordetellapertussis百日咳杆菌endogenous infection 内源性感染borrelia 疏螺旋体属el-tor biotype 埃尔托生物型b.recurrentis 回归热螺旋体 endotoxin 内毒素b.vincenti 奋森氏螺旋体enteric cytopathogenic humanorpan virus,埃可病毒b.burdorfe 莱姆病螺旋体 enterobacteriaceae 肠粘附性大肠杆菌botulism 肉毒中毒enterohemorrhagic escherichiacoli,etec肠产毒性大肠杆菌brucella 布氏杆菌属enterovirus 肠道病毒br.bovis 牛布氏杆菌envelope 囊膜br.melitensis 羊布氏杆菌enteropathoginc escherichiacoli,epec肠致病性大肠杆菌br.suis 猪布氏杆菌epidermophyton 表皮癣菌属bunyaviridae 布尼安病毒科 epstein-barr virus,eb virus eb eb病毒calmett-guerinsvaccine卡介苗escherichia 埃希氏菌属campylobacter 弯曲菌属 e.coli 大肠杆菌c.jejuni 空肠弯曲菌exogenous infection 外源性感染candidaalbicans白色念珠菌exotoxin 外毒素capsid 衣壳fimbriae 纤毛capsomere 壳微粒flagellum 鞭毛capsule 荚膜flaviviridae 黄病毒科carrier 带菌者fungus 真菌cell culture 细胞培养fusion 融合cell fusion 细胞融合ganciclovir 丙氧鸟苷cell wall 细胞壁genetic engineering 基因工程chitin 甲壳质genetic recombination 基因重组chlamydiae 衣原体growth inhibition test 生长抑制实验chlamydiapneumonia肺炎衣原体guarneiri body 顾氏小体hantaan virus 汉滩病毒horizontal tansmission 水平传播hantavirus 汉坦病毒属human immunodeficiency virus,hiv 人免疫缺陷病毒(艾滋病病毒)halophilicvibrio嗜盐弧菌hyaluronidase 透明质酸酶hbcag 乙型肝炎病毒immunofluorescence technic,ift 免疫荧光法核心抗原 hbeag 乙型肝炎病毒e 抗原inapparent imfection隐性传染 hbsag 乙型肝炎病毒表面抗原inclusion body包涵体hbv dna polymerase 乙型肝炎病毒dna 聚合酶 infectious mononucleosis传染性单核细胞增多症 heat-labiletoxin,lt 不耐热肠毒素 inferferon(ifn)干扰素 heat-stabletoxin,st耐热肠毒素 integrated infection整合传染 hela cells,hela 细胞 invasiveness 侵袭力 hemagglutinin,ha血凝集japanese encephalitis virus,jev 日本脑炎病毒hemagglutination-inhibition test血凝抑制试验 japanese b encephalitis,jbe 日本乙型脑炎hemagglutinati on phenomenon 血凝现象 klebsiella 克雷伯氏菌属 hemolysin 溶血素 k.pneumoniae 肺炎克雷伯氏菌hemlphilus 嗜血杆菌属 lactobacillus乳杆菌属 h.influenzae 流行性感冒杆菌latent infection潜伏感染hemorrhagicfever with renal syndrome 肾综合征合征出血热ld50半数致死量helicalsymmetry螺旋对称legionella 军团菌属 hepadnaviridae 嗜肝病毒科 legionnaires disease 军团菌病 hepatitis avirus,hav 甲型肝炎病毒 lentivirinae慢病毒科 hepatitis bvirus,hbv乙型肝炎病毒 leptospira钩端螺旋体属 hepatitis virus 肝炎病毒 local infection 局部感染 herpes simplexvirus单纯疱疹病毒 lipopolysaccharide,lps脂多糖 varicella-zostervirus带状疱疹病毒 lysogenic bacteria溶原性细菌 herpetoviridae 疱疹病毒科 lysogeny 溶原状态 human herps virus type 6、7 人疱疹病毒6、7型lysogenic phage溶原性噬菌体 histoplasma capsulatum 荚膜组织胞浆菌lyssavirus狂犬病毒科measles virus 麻疹病毒papovaviridae 乳多空病毒科mesosome 中介体parainfluenza virus 副流感病毒metabolicinhibition test代谢抑制试验 parmyxoviridae 副粘病毒科metachromaticgranules异染颗粒paramyxovirus 副粘病毒microbiology 微生物学parvoviridae 微小病毒科microcapsule 微荚膜parvavirus 微小病毒mcrococcustetragenus四联球菌penicillium 青霉菌micrometer,micron(u=um)微米penetration 穿入microorganism 微生物peplomer 囊微粒mold 霉菌persistent infection 持续性感染moniliaalbicans白色念珠菌pesticine-1 鼠疫杆菌杀菌素mucor 毛霉菌picornaviridae 小核糖核酸病毒科mumps virus 腮腺炎病毒pilus(或fimbrae) 菌毛mutation 突变plague 鼠疫mycelium 菌丝体plaque forming unit,pfu 空斑形成单位mycobacterium 分枝杆菌属plasmid 质粒m.tuberculosis 结核分枝杆菌 plasmin 胞浆素mycolic acid 分枝菌酸poliovirus 脊髓灰质炎病毒mycoplasma 支原体属polykaryocyte 多核巨细胞mycostatin 制霉菌素poly i:c,polyinosinic:cytrdyeic acid 多聚肌苷酸:胞苷酸mycotoxicosis 真菌毒素中毒症poxviridae 痘病毒科mycrosporum 小孢子癣菌属 poxvirus 痘病毒nairovirus 内罗病毒属pre-s antigen 前s抗原negri body 内基氏小体primary cell culture 原代细胞培养neisseria 奈瑟氏菌属prickle cell 棘细胞n.gonorrhoeae 淋病奈瑟氏力 prion 蛋白侵染因子n.meningitidis 脑膜炎奈瑟氏菌β-propialactone β-丙内脂newenterovirus新型肠道病毒 prophage 前噬菌体normal flora 正常菌群proteus 变形杆菌nuclearmaterial核质protoplast 原生质体nucleocapsid 核衣壳provirus 前病毒oldtuberculin,ot旧结核菌素pseudomonas 假单胞菌属oncornavirusrna肿瘤病毒p.aeruginosa 绿脓杆菌orthomyxoviridae正粘病毒科purified protein derivative,ppd 纯化蛋白衍生物orthomyxovirus正粘病毒pyosepticemia 脓毒血症humanpapilloma virus乳头瘤病毒pyrogen 热原质rabies virus 狂犬病病毒sh.boydii 鲍氏志贺氏菌receptorbinding protein受体连接蛋白 sh.dysenteriae 痢疾志贺氏菌release 释放sh.flexneri 福氏志贺氏菌reoviridae 呼肠孤病毒科 sh.sonnei 宋内氏志贺氏菌replication 复制slow infection 慢发性感染resistancefactor耐药因子spheroplast 原生质球resistance transfer factor,ptf 耐药性传递因子spike 刺突respiratorysyncytialvirus,rs virus呼吸道病毒spirillum 螺菌respiratoryviruses逆转录病毒科 spore 芽胞,孢子retroviridae 逆转录病毒staphylococcus 葡萄球菌retrovirus 逆转录酶stap.aureus 金黄色葡萄球菌reversetranscriptase弹状病毒科stap.epidermidis 表皮葡萄球菌rhabdoviridae 病毒唑stap.protein a,spa 葡萄球菌a蛋白ribavirin(virozol)rna病毒sterdy state infection 稳定感染rickettsia 立克次氏体属 sterillization 灭菌rifampin 利福平streptococcus 链球菌属ringworm fungi 癣菌strep.anhemolyticus 非溶血性链球菌rochalimaea 罗沙利马体属 strep.hemolyticus 溶血性链球菌(乙型链球菌)rotavirus 轮状病毒strp.pneumonia 肺炎链球菌russianspring-summer encephalitis virus 苏联春夏季脑炎病毒strep.viridans 草绿色链球菌(甲型链球菌)sabourauds medium 沙保氏培养培养基streptodornase 链道酶salmonella 沙门氏菌属streptokinase 链激酶s.choleraesuis 猪霍乱沙氏菌 subclinical infection 亚临床感染s.typhi 伤寒沙门氏菌 subunit vaccine 亚单位疫苗s.paratyphi 副伤寒沙门氏subacute sclerosing panencephalitis 亚急性硬化性全脑炎菌sspes.typhimurium 鼠伤寒沙门氏菌subvirus 亚病毒sarcina 八迭球菌syncytium 合胞体shigella 志贺菌属syphilis 梅毒tcid50 50% 组织细胞感染量systemic infection 全身感染teichoic acid 磷壁酸vertical transmission 垂直传播temperatephage温和噬菌体vibrio 弧菌属temperature-sensitive conditional mutant,ts 温度敏感突变株v.cholerae 霍乱弧菌thymidinekinase胸腺核苷激酶 veillenella 韦荣氏球菌属togaviridae 披膜病毒科virino 朊病毒toxemia 毒血症virion 病毒体toxin 类毒素viroid 类病毒toxoid 密螺旋体属virulence 毒力treponema 梅毒螺旋体virulent phage 毒性噬菌体t.pallidum 雅司螺旋体virus 病毒t.pertenue 毛癣菌属virusoid 拟病毒trichophyton 毛癣菌素v—onc 病毒癌基因trichophytin 脱壳widal test 肥达氏试验uncoating 脲原体属yersinia 耶尔森氏菌属ureaplasma 脲原体属y.pestis 鼠疫杆菌vaccine 疫苗y.pseudotuberculosis 假结核杆菌。
小儿呼吸道合胞病毒感染

小儿呼吸道合胞病毒感染内容摘要:呼吸道合胞病毒是婴幼儿呼吸道感染最常见的病原体。
近年研究发现,其发病机制包括病毒与宿主受累细胞损伤、炎症、体液和局部免疫反应及高反应性之间的相互作用。
呼吸道分泌物荧光素标记抗体检测快速,敏感性和特异性高。
三氮唑核苷、免疫球蛋白及干扰素常能达到早期病因治疗,通过气雾吸入等局部给药途径,使疗效明显提高。
尚在试制的呼吸道合胞病毒新型疫苗及基因治疗研究为该病的防治提供了良好的前景。
呼吸道合胞病毒是婴幼儿呼吸道感染最常见的病原体。
近年研究发现,其发病机制包括病毒与宿主受累细胞损伤、炎症、体液和局部免疫反应及高反应性之间的相互作用。
呼吸道分泌物荧光素标记抗体检测快速,敏感性和特异性高。
三氮唑核苷、免疫球蛋白及干扰素常能达到早期病因治疗,通过气雾吸入等局部给药途径,使疗效明显提高。
尚在试制的呼吸道合胞病毒新型疫苗及基因治疗研究为该病的防治提供了良好的前景。
呼吸道感染;呼吸道合胞病毒;婴幼儿;诊断;治疗呼吸道合胞病毒(respiratorysyncytialvirus,RSV)属副黏病毒科肺炎属,Morris从一只有感冒症状的实验动物黑猩猩的鼻咽分泌物中分离出第一株RSV。
Chanock先后从Baltimore 市2例分别患肺炎和有喘息症状患儿的咽拭子中分离到。
因其在组织培养中能形成特殊的细胞融合病变而得名。
呼吸道合胞病毒是婴幼儿呼吸道感染最常见的病原体,特别是2~6个月小婴儿RSV感染后常发生严重毛细支气管炎和肺炎。
通常在冬、春季节流行。
在世界不同地区每年因RSV感染而需住院治疗的患儿为1‰~5‰,住院病人病死率为1‰~3‰[1]。
由此可见RSV感染是严重危害小儿生命健康的一种疾病。
近年来对该病的研究有了较大进展,现做一综合介绍。
1RVS感染的发病机制及临床特征至今已鉴定RSV有2个主要血清型和9个亚型,常常通过托儿机构、家庭和其他公共场所造成传播,亦是引起住院小儿医院内感染的常见原因。
酵母菌色氨酸组氨酸腺嘌呤合成缺陷型(SD-TRPHISADE
产品成分
酵 母 菌 色 氨 酸 / 组 氨 酸 / 腺 嘌 呤 合 成 缺 陷 型 培 养 基 ( Synthetic Dropout Medium-Tryptophan/Histidine/Adenine)含有除色氨酸、组氨酸和腺嘌呤外其它主要氨基酸基本营养元素(见 产品配方表),同时包括基础酵母氮源成分和葡萄糖碳源成分等。
用量(毫克/升) 0 0 20 0 0 0 0 0 0 0 0 0 30 30 20 0 50 0 0 200 0 30 20 0
2
产品内容
培养基(Reagent A) 产品说明书
毫升 1份
保存方式
保存在室温下,避免光照,有效保证 6 月
实验提示
1. 直接加入适量的培养基(Reagent A)到目标酵母细胞里 2. 可用于特定酵母细胞株的选择培养
注意事项
1. 本产品为 100 毫升规格 2. 可用于特定酵母细胞株的选择培养 3. 本产品使用葡萄糖作为碳源成分 4. 使用时,须无菌操作 5. 使用时,避免污染母液 6. 本公司提供系列酵母培养TRP/HIS/ADE)培养基 产品说明书(中文版)
主要用途
酵 母 菌 色 氨 酸 / 组 氨 酸 / 腺 嘌 呤 合 成 缺 陷 型 培 养 基 ( Synthetic Dropout Medium-Tryptophan/Histidine/Adenine)是一种旨在用于选择性支持特定酵母细胞株生长,探测其细胞表现 型的最基本营养液。其标准化成分中去除色氨酸、组氨酸和腺嘌呤成分,适合特定酵母菌的培养,排除色 氨酸、组氨酸和腺嘌呤依赖性细胞株的存在。产品即到即用,严格无菌、无病毒和支原体,性能稳定,促 特定细胞生长效应出色,建立标准化体系。
质量标准
1. 本产品经鉴定性能稳定 1
呼吸道合胞病毒
形态学
呼吸道合胞病毒病毒形态为球形,直径为120~300nm, 有包膜,基因组为非分节段的单负链RNA,主要编码 10种蛋白质,即融合蛋白(F)、黏附蛋白(G)和小疏水蛋白(SH)三种跨膜蛋白,两种基质蛋白M1和M2,三种 与病毒RNA相结合形成核衣壳的蛋白(N、P和L),两种非结构蛋白(NS1和NS2)。病毒包膜上有糖蛋白组成的刺 突,无HA、NA和HL 。
呼吸道合胞病毒
副黏病毒科肺病毒属病毒
01 形态学
03 流行病学
目录
02 病毒抗性 04 致病机制
05 病原检验
07 药物研究
目录
06 预防与治疗 08 疫苗研发
呼吸道合胞病毒(Respiratory syncytial virus, RSV)属于副黏病毒科的肺病毒属(Pneumovirus), 只有一种血清型。主要引起6个月以下婴儿患细支气管炎和肺炎等下呼吸道感染,以及较大儿童和成人的鼻炎、感 冒等上呼吸道感染。
病原检验
常用免疫荧光试验等直接检查咽部脱落上皮细胞内的RSV抗原,以及RT-PCR检测病毒核酸等进行辅助诊断。
因RSV所致疾病在临床上与其他病毒或细菌所致类似疾病难以区别,故进行病毒分离和抗体检测是必要的, 即使操作复杂、费时。
近年来利用鼻咽分泌物脱落细胞及血清中IgM抗体的间接法免疫荧光技术,ELISA,碱性磷酸酶抗碱性磷酸酶 桥联酶标法(APAAP),生物素抗生物素ELISA法,辣根过氧化物酶—抗辣根过氧化物酶法(PAP)、单克隆抗体 荧光法等都能进行合胞病毒感染的快速诊断。
致病机制
RSV感染仅引起轻微的呼吸道纤毛上皮细胞损伤,但在2~6个月的婴幼儿感染中,可引起细支气管炎和肺炎等 严重呼吸道疾病,其发生机制除病毒感染直接作用外,可能与婴幼儿呼吸道组织学特性、免疫功能发育未完善及 免疫病理损伤有关。
住院医生规范化培训英语单词
astrointestinal perforation胃肠穿孔erythema 红斑active treatment积极疗法echocardiography超声心动图acoustic window 声窗emphysema肺气肿Angiodysplasia 血管发育畸形enteroclysis 小肠造影advance cancer 晚期癌enteroscopy 肠镜检查autopsy尸检ectopic pregnancy异位妊娠antidepressant抗抑郁药epidemicn. 传染病acute abdomen急腹症endoscopic内窥镜检查的atherosclerosis动脉粥样硬化evidence-based medicine 循证医学aerobic exercise有养运动etiology病原学arthritis关节炎glandular lumina 腺腔appendicitis阑尾炎growth factor生长因子arterial oxygen tension动脉血养分压genetic information遗传信息asthma哮喘genetic diseases遗传疾病antibiotic抗生素genetic markers基因标记bioartificial liver生物人工肝genetics遗传学biopsy specimen 活检标本genomics基因组学blood culture血培养gallbladder 胆囊breast carcinoma 乳腺癌Gallstones胆石bacterial pneumonia细菌性肺炎hypopnea 呼吸减弱biopsy活组织检查human genome project人类基因组计划biliary tree 胆道系统health care 卫生保健cerebrovascular accident脑血管意外hypertension高血压coronary angiography冠脉造影Hyperplasia 超常增生carbohydrate糖类hypopnea 呼吸减弱carcino-embryonic antigen癌胚抗原hyperventilation 换气过度combination therapy联合治疗heart and lung failure 心肺衰竭clinical trials 临床试验inflammation炎症complication并发症influclinical reasoning 诊断Cytochrome细胞色素intensive treatment 强力疗法chest radiogrph 胸片intestinal ischemia肠缺血cholecustectomy胆囊切除术initial history 原始病史clinical setting临床环境lung compliance肺顺应性clinician 临床医师laparoscopy腹腔镜检查术clinical evaluation临床评价lassification 分类clinical course临床过程macromolecules 大分子蛋白colonoscopy 结肠镜malignancy恶性肿瘤dyspnea 呼吸困难motion artifact运动伪差diastolic pressure舒张压microscopic examination显微镜检查dizziness头晕myocardial infarction心肌梗死mortality 死亡数、死亡率osteoarthrits骨关节炎nostril鼻孔ovarian cyst卵巢囊肿Nucleic Acid核苷酸pelvis骨盆,肾盂prostate-specific antigen (PSA)前列腺特异性抗原prostate serum acid phosphatase(PSAP) 血清酸性磷酸酶Pharmacological 药理学sphygmomanometer 血压计Proteolytic 蛋白水解的stem cell干细胞prostate 前列腺symptom症状prostatic 前列腺的small intestine小肠peritoneal cavity腹膜腔stratification 层化成层pleural space胸膜腔symptoms症状progenitor cell祖细胞telangiectasia 毛细血管扩张peptic ulcer消化性溃疡tubo-ovarian abscess输卵管-卵巢脓肿perfusion 灌注total parenteral nutrition全胃肠外营养palliative姑息性治疗transfusion 输血pneumonia 肺炎tomographic DSA体层摄影数字减影血管造影physical examination体格检查threshold concentration阈浓度primary tumor原发性肿瘤tumor marker肿瘤标记物primary site基本位点ureteral calculi输尿管结石pathologic病理学的underlying cancer 潜在癌pneumo peritoneum气腹percutaneous drainage孔经皮穿刺引流placebo安慰剂visceral内脏的prostatitis 前列腺炎viscus (复viscera) 内脏puncture 穿刺renal肾脏的rebound tenderness反跳痛respiratory illness呼吸系疾病respiratory syncytial viruses呼吸道合胞病毒radiographic X线照相术的rationale 基本原理replacement therapy代替治疗radiologic 放射学的regimen 养生法、食物疗法astrointestinal perforation胃肠穿孔erythema 红斑active treatment积极疗法echocardiography超声心动图acoustic window 声窗emphysema肺气肿Angiodysplasia 血管发育畸形enteroclysis 小肠造影advance cancer 晚期癌enteroscopy 肠镜检查autopsy尸检ectopic pregnancy异位妊娠antidepressant抗抑郁药epidemicn. 传染病acute abdomen急腹症endoscopic内窥镜检查的atherosclerosis动脉粥样硬化evidence-based medicine 循证医学aerobic exercise有养运动etiology病原学arthritis关节炎glandular lumina 腺腔appendicitis阑尾炎growth factor生长因子arterial oxygen tension动脉血养分压genetic information遗传信息asthma哮喘genetic diseases遗传疾病antibiotic抗生素genetic markers基因标记bioartificial liver生物人工肝genetics遗传学biopsy specimen 活检标本genomics基因组学blood culture血培养gallbladder 胆囊breast carcinoma 乳腺癌Gallstones胆石bacterial pneumonia细菌性肺炎hypopnea 呼吸减弱biopsy活组织检查human genome project人类基因组计划biliary tree 胆道系统health care 卫生保健cerebrovascular accident脑血管意外hypertension高血压coronary angiography冠脉造影Hyperplasia 超常增生carbohydrate糖类hypopnea 呼吸减弱carcino-embryonic antigen癌胚抗原hyperventilation 换气过度combination therapy联合治疗heart and lung failure 心肺衰竭clinical trials 临床试验inflammation炎症complication并发症influclinical reasoning 诊断Cytochrome细胞色素intensive treatment 强力疗法chest radiogrph 胸片intestinal ischemia肠缺血cholecustectomy胆囊切除术initial history 原始病史clinical setting临床环境lung compliance肺顺应性clinician 临床医师laparoscopy腹腔镜检查术clinical evaluation临床评价lassification 分类clinical course临床过程macromolecules 大分子蛋白colonoscopy 结肠镜malignancy恶性肿瘤dyspnea 呼吸困难motion artifact运动伪差diastolic pressure舒张压microscopic examination显微镜检查dizziness头晕myocardial infarction心肌梗死mortality 死亡数、死亡率osteoarthrits骨关节炎nostril鼻孔ovarian cyst卵巢囊肿Nucleic Acid核苷酸pelvis骨盆,肾盂prostate-specific antigen (PSA)前列腺特异性抗原prostate serum acid phosphatase(PSAP) 血清酸性磷酸酶Pharmacological 药理学sphygmomanometer 血压计Proteolytic 蛋白水解的stem cell干细胞prostate 前列腺symptom症状prostatic 前列腺的small intestine小肠peritoneal cavity腹膜腔stratification 层化成层pleural space胸膜腔symptoms症状progenitor cell祖细胞telangiectasia 毛细血管扩张peptic ulcer消化性溃疡tubo-ovarian abscess输卵管-卵巢脓肿perfusion 灌注total parenteral nutrition全胃肠外营养palliative姑息性治疗transfusion 输血pneumonia 肺炎tomographic DSA体层摄影数字减影血管造影physical examination体格检查threshold concentration阈浓度primary tumor原发性肿瘤tumor marker肿瘤标记物primary site基本位点ureteral calculi输尿管结石pathologic病理学的underlying cancer 潜在癌pneumo peritoneum气腹percutaneous drainage孔经皮穿刺引流placebo安慰剂visceral内脏的prostatitis 前列腺炎viscus (复viscera) 内脏puncture 穿刺renal肾脏的rebound tenderness反跳痛respiratory illness呼吸系疾病respiratory syncytial viruses呼吸道合胞病毒radiographic X线照相术的rationale 基本原理replacement therapy代替治疗radiologic 放射学的regimen 养生法、食物疗法。
2024年关于呼吸道合胞病毒感染治疗和预防
2024年关于呼吸道合胞病毒感染治疗和预防的文章呼吸道合胞病毒(Respiratory Syncytial Virus, RSV)是一种全球范围内普遍存在的病原体,尤其在婴幼儿和老年人中引起严重的呼吸道感染。
本文将探讨2024年RSV感染的治疗和预防策略,以及最新的研究进展。
1. RSV感染的治疗RSV感染的治疗主要以支持性护理和对症治疗为主。
目前,全球尚无专门针对RSV感染的有效治疗方法。
支持治疗包括及时补液、保持气道通畅和氧疗,以呼吸频率、氧饱和度、动脉血气分析为指导。
临床上常使用支气管舒张剂、糖皮质激素和白三烯受体拮抗剂等作为对症治疗。
雾化吸入利巴韦林经美国食品药品监督管理局(FDA)批准用于治疗住院儿童严重RSV下呼吸道感染相关的药物。
在中国,口服和注射给药均被批准用于RSV的治疗,但因其安全性和有效性仍存在争议,目前仅用于存在免疫功能受损/缺陷的高危RSV感染患儿。
2.RSV感染的预防预防RSV感染的策略包括非药物干预和药物预防。
非药物干预在家庭中最常见的预防方式就是戴口罩、常洗手,尽量少接触或者不接触感染者。
对于6个月以内的孩子,要采取“蚕茧式”保护。
药物预防方面,长效单克隆抗体尼塞韦单抗(Nirsevimab)是我国目前唯一获批的用于预防婴儿RSV感染的免疫制品,预计于2024-2025年RSV流行季在我国上市。
3.RSV感染的季节性和监测RSV流行在全球大部分地区呈现出明显的季节性,全面了解RSV季节性的地区差异对于确定RSV免疫规划的时机至关重要。
中国的研究系统地描述了RSV季节性的地区分布,划分了传播区域,并评估了RSV季节性预防策略在中国的潜在适用性。
研究通过系统综述检索相关文献及监测报告,提取RSV流行相关数据,对中国RSV季节性流行进行系统分析。
4.RSV疫苗的研发2024年,我国首款国产RSV疫苗正式启动I/II期临床试验,这标志着我国在RSV 疫苗研发领域迈出了重要一步。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Respiratory Syncytial Virus Matrix Protein Induces Lung Epithelial Cell Cycle Arrest through a p53Dependent PathwayTao Bian1,John D.Gibbs2,Claes O¨rvell3,Farhad Imani4*1Laboratory of Respiratory Biology,National Institute of Environmental Human Science,Durham,North Carolina,United States of America,2Global Vaccines,Inc., Durham,North Carolina,United States of America,3Huddinge University Hospital,Department of Clinical Virology,Karolinska Institute,Stockholm,Sweden,4ViraSource Laboratories,Durham,North Carolina,United States of AmericaAbstractRespiratory syncytial virus(RSV)is the major cause of viral respiratory infections in children.Our previous study showed that the RSV infection induced lung epithelial cell cycle arrest,which enhanced virus replication.To address the mechanism of RSV-induced cell cycle arrest,we examined the contribution of RSV-matrix(RSV-M)protein.In this report,we show that in both the A549cell line and primary human bronchial epithelial(PHBE)cells,transfection with RSV-M protein caused the cells to proliferate at a slower rate than in control cells.The cell cycle analysis showed that RSV-M protein induced G1phase arrest in A549cells,and G1and G2/M phase arrest in PHBE cells.Interestingly,RSV-M expression induced p53and p21 accumulation and decreased phosphorylation of retinoblastoma protein(Rb).Further,induction of cell cycle arrest by RSV-M was not observed in a p53-deficient epithelial cell line(H1299).However,cell cycle arrest was restored after transfection of p53cDNA into H1299cells.Taken together,these results indicate that RSV-M protein regulates lung epithelial cell cycle through a p53-dependent pathway,which enhances RSV replication.Citation:Bian T,Gibbs JD,O¨rvell C,Imani F(2012)Respiratory Syncytial Virus Matrix Protein Induces Lung Epithelial Cell Cycle Arrest through a p53Dependent Pathway.PLoS ONE7(5):e38052.doi:10.1371/journal.pone.0038052Editor:Steven M.Varga,University of Iowa,United States of AmericaReceived January27,2012;Accepted May2,2012;Published May25,2012Copyright:ß2012Bian et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use,distribution,and reproduction in any medium,provided the original author and source are credited.Funding:This research was fully funded by Intramural National Institute of Health(NIH)funding.The funder had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.Competing Interests:John Gibbs is an employee of Global Vaccines,Inc.and Farhad Imani an employee of ViraSource Laboratories.There are no patents, products in development or marketed products to declare.This does not alter the authors’adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.*E-mail:fimani@IntroductionRespiratory syncytial virus(RSV)is a major cause of respiratory tract infection in infants and young children worldwide[1,2].In the US,approximately125,000children are hospitalized annually with a2%of mortality rate[3].However,there are no effective vaccines for RSV.Importantly,RSV infection in early life has been associated with subsequent development and exacerbations of asthma[4,5].RSV,a member of paramyxoviridae family,is an enveloped virus with a single-stranded negative sense RNA genome,which replicates in the cytoplasm of host cells.The RSV genome encodes nine structural proteins and two non-structural proteins,compris-ing the envelope glycoproteins(F,G,and SH),the nucleocapsid proteins(N,P,and L),the nucleocapsid-associated proteins(M2-1 and M2-2),and the matrix protein(M)[6].In our previous study we showed that RSV infection induced epithelial cell cycle arrest[7].In addition to participating as an integral part the virus particle,RSV-M protein is shuttled to the host cell nucleus at an early stage of virus replication,where it can inhibit cellular transcription[8,9,10,11].Therefore,we hypothe-sized that RSV-M protein played a key role in the RSV-induced cell cycle inhibition and therefore enhanced virus replication.To further delineate the mechanism of RSV-M protein induction of cell cycle arrest,we examined the contribution of the tumor suppressor protein p53,which plays a pivotal role in cell cycle arrest[12].Once activated,p53binds cellular DNA and induces expression of several genes including GADD45,IGF-BP3 and WAF1/CIP1which encodes p21.p21is a key molecule in cell cycle regulation binds to and inhibits the activity of cyclin-dependent kinase(CDK)complexes,thereby inhibiting Rb phosphorylation.The phosphorylation of Rb is a well-described regulator of the cell cycle.Therefore,over expression of p53causes arrest of cell growth[13].Virus infections are known to activate the p53pathway. Infection of influenza A virus,Epstein–Barr virus(EBV), adenovirus,HIV-1and minute virus induce host cell accumulation of p53,cell cycle arrest and apoptosis[14,15,16,17,18,19,20]. Over-expression of p53also is observed in chronic hepatitis C virus infected patients,suggesting an impaired cell cycle pro-gression that lead to worsening of the disease[21].However,some viral proteins,particularly in DNA viruses,form complexes with p53and reduce effective p53levels[22].Our previous study showed that RSV infection induced lung epithelial cell cycle arrest which subsequently enhanced RSV replication[7]. Furthermore,viral proteins such as non-structural protein of influenza A virus,large T antigen of the human polyoma virus, JC virus large T protein,human parvovirus B19NS1protein and avian reovirus p17protein induce cell cycle arrest in G1or G2phase through p53or p21accumulation[23,24,25,26,27].Interestingly,the fusion protein of RSV(RSV-F)triggers p53-dependent apoptosis[25].In our current study,our data showed that RSV-M protein induced lung epithelial cell cycle arrest through the accumulation of p53protein.Material and MethodsPlasmid Construction and TransfectionThe eukaryotic expression vector pSG-5was purchased from Stratagene(Santa Clara,CA).The RSV-M protein gene open reading frame(ORF)was amplified from RNA isolated from RSV-A2-infected cells by RT-PCR with the forward primer:59-TGC GGATCC ATGGAAACATACGTGAAC-39;the reverse primer:59-TGC GGATCC TTAATCTTCCATGGGTTTG-39. After digestion with restriction enzyme BamHI,the RSV-M gene was cloned into pSG-5(pSG-M)using standard techniques. Sequencing was performed to verify the fidelity on the cloned cDNA.pcDNA3.1-p53plasmid expressing the wild type human p53was kindly provided by Dr.Michael Resnick(NIEHS,NIH). For transfections,DOTAP liposomal transfection reagent(Roche, Indianapolis,IN)was used for transfection DNA into A549cells. Nucleofector kit(Lonza,Walkersville,MD)was used for trans-fection into PHBE cells with program W-001according to the manufacturer’s instructions.The plasmid expressing GFP (pmaxGFP)(Lonza,Walkersville,MD)was used to evaluate transfection efficacy.Cells and Cell Culture ReagentsPrimary human bronchial epithelial(PHBE)cells and serum-free bronchial epithelial basal medium with growth supplements were purchased from Lonza(Walkersville,MD).The human alveolar epithelial cell line A549and human p53-deficient lung cancer cell line H1299were purchased from American Type Culture Collection(ATCC Number:CCL-185&CRL-5803), which were grown as a monolayer in Dulbecco’s modified Eagle’s/ Ham’s F-12medium with5%fetal calf serum and1%penicillin-streptomycin at37u C in a5%CO2humidified incubator.H1299 is a human lung lymph node-derived non-small cell carcinoma cell line which has a homozygous partial deletion of the TP53gene and does not express p53protein.The p53expressing H1299 (p53+/+)cell line was derived from H1299after transfection with pcDNA3.1-p53plasmid.G418(Sigma-Aldrich,St.Louis,MO) selection was used to isolate stably expressing cells with the wild type p53protein.Nutlin-3,a cell cycle regulatory compound through accumulation of p53,was purchased from Sigma-Aldrich (St.Louis,MO),diluted in captisol(CyDex,Kansas City,MO), and was used at of10m M.Virus and InfectionsThe human RSV subtype A2was grown and titrated by standard plaque assay in human epithelial cell line HEp-2as previously described[28].After cells reached70%confluency, they were infected with RSV at the indicated multiplicity of infection(MOI).Cells were then harvested at various times and were used for analysis.Cell Proliferation Assay and Cell Cycle AnalysisFor cell proliferation assays,A549and PHBE cells were transfected with pSG-M,and pSG-5as vector control.Cells were treated with nutlin-3as positive control for p53-induced cell cycle arrest.Cells were treated captisol alone as the vehicle control.At indicated time-point post-treatment,cells were washed and resuspended in phosphate-buffered saline(PBS). Total cell numbers at each day were determined with a hemocytometer,and dead cells were identified by trypan blue staining.Cell cycle analysis was performed by flow cytometry.Briefly,4hr prior to harvesting cells were treated with10m M bromodeoxyuridine(BrdU)(BD Bioscience,San Jose,CA).Cells were then fixed with70%ethanol for30min at220u C and then labeled with fluorescein isothiocyanate-conjugated anti-BrdU antibody according to the manufacturer’s instructions(BD Biosciences,San Jose,CA).The cell cycle properties were then analyzed using BrdU incorporation and7-Amino-actinomycin D(7-AAD)(BD Biosciences,San Jose,CA) staining.Flow cytometry was carried out on a Becton-Dickinson FACSort flow cytometer,and quantification of cell cycle distribution was determined using either CellQuest or Modfit software(BD Biosciences,San Jose,CA).Western Blot and AntibodiesFor western blot analysis,cells were washed twice with PBS and were then lysed in Laemmli sample buffer containing2.5%b-mercaptoethanol.The total cellular proteins were denatured and reduced by heating at95u C for5min.Protein concentration was then determined by the Bio-Rad protein assay(Bio-Rad,Hercules, CA).Proteins were resolved by SDS-polyacrylamide gel electro-phoresis and were electrotransferred onto nitrocellulose mem-branes.The membranes were probed with respective primary antibodies and horseradish peroxidase-conjugated secondary antibody according to the manufacturer’s instructions.Primary antibodies were anti-p53(DO-1),anti-Phospho-p53(serine-15), anti-p21,anti-phospho-Rb(p780)and anti-glyceraldehyde-3-phosphate dehydrogenase(anti-GAPDH)(Cell Signaling,Dan-vers,MA);anti-RSV(Fitzgerald Industries,Concord,MA). Monoclonal antibody C781is specific for M protein[9].The enhanced chemiluminescence(ECL)western blot detection system was then used to visualize the immunoblotted proteins(GE Healthcare,Piscataway,NJ).Quantification of the bands were performed using ImageJ software.Quantitative RT-PCRTotal RNA was isolated with TRIzol RNA isolation reagent (Invitrogen,Carlsbad,CA)following the manufacturer’s instruc-tions.RNA was reverse transcribed into first-strand cDNA using Superscript reverse transcriptase(Invitrogen,Carlsbad,CA)with oligo(A)primer.The cDNA was then amplified by real-time PCR (RT-PCR)as previously described[7].All RT-PCR results were normalized according to GAPDH before analysis.Primer sequences used in RT-PCR were as follows:GAPDH(forward) 59-GGACCTGACCTGCCGTCTAG-39(reverse)59-TAGCC-CAGGATGCCCTTGAG-39;RSV-NS1(forward)59-AGA-GATGGGCAGCAATTCAT-39(reverse)59-CACAAACA-CAATGCCATTCA-39;RSV-NS2(forward)59-ATCAATTCAGCCAACCCAAC-39(reverse)59-ATGTGGCCTGTTTTTCATCA-39;RSV-P(forward)59-GGCAAGACTCAGGAATGAGG-39(reverse)59-GTTGGAT-GATTGGGTTGGTT-39;RSV-M(forward)59-AAAGACGAT-GACCCTGCATC-39(reverse)59-TGGTAAATTTGCTGGG-CATT-39;RSV-G(forward)59-CAACCCCAACATACCTCACC-39(reverse)59-GTGGATTG-CAGGGTTGACTT-39;RSV-N(forward)59-AGGATTGTT-TATGAATGCCTATGGT-39(reverse)59-GCTTTTGGGTTGTTCAATATATGGTAC-39;RSV-L(for-ward)59-CAGCCAAATCCAACCAACTT-39(reverse)59-AATTCCCTGCTCCTTCACCT-39and p53(forward)59-TCAACAAGATGTTTTGCCAACTG-39(reverse)59-ATGTGCTGTGACTGCTTGTAGATG-39.Statistical AnalysisStatistical analysis was performed on cell proliferation assays,cell cycle analysis,quantification of bands in western blots,RT-PCR and plaque assays.The values are standard errors of the means based on Student’s t test.Significance values (P values)of less than 0.05were interpreted as statistically significant.ResultsRSV-M Protein Expression Inhibits Epithelial Cells ProliferationTo assess the role of RSV-M in cell cycle regulation,we first determined the expression of RSV-M in A549and PHBE cells by western blot analysis (Fig.1A).The data showed that a protein of approximately 29KDa was expressed after transfection,which reacted with a specific mAb to RSV-M protein [10].The transfection efficiencies are 90–100%in A549cell and 70–80%in PHBE according to the GFP expressing plasmid (data not shown).Next,to determine the effect of RSV-M protein expression on cell proliferation,we transfected both A549and PHBE cells with the vector alone or with the vector carrying RSV-M.After indicated times post transfection,cells were quantified by enumeration using a hemocytometer (Fig.1B).The results showed that pSG-M transfected cells replicated at a significantly slower rate than cells transfected with the vector alone (pSG-5)or treated with the vehicle control (captisol)(Fig.1B).The cells treated with nutlin-3were used as a positive control,which have similar proliferation rate as pSG-M transfected cells.The number of deadcells did not have significant difference,and were ,1%in all samples.M Protein Expression Induces Cell Cycle ArrestSince there was a significant reduction in cell proliferation,we next performed cell cycle analysis using BrdU incorporation and flow cytometry.Data showed that in A549cells pSG-M trans-fection induced a significant G1phase arrest (G1phase:51.462.2%vs.44.761.3%)(Fig.2A).However,in PHBE cells,both G1and G2/M phase arrest were observed (G1phase:67.160.5%vs.54.561.0%;G2phase:15.561.2%vs.8.660.9%)(Fig.2B).The data showed that RSV-M alone was sufficient for induction of cell cycle arrest.M Protein Expression Activates the p53PathwayTo first determine whether RSV infection induced p53we performed a kinetic study in infected A549cells.Western blot analysis showed that RSV infection induced p53in a time dependent manner (Fig.3A).This induction,we reasoned,maybe in part due to RSV-M protein expression.To investigate whether p53induction and activation of downstream signaling molecules was in part due to RSV M expression,we performed transfection studies.A549cells and PHBE cells (Fig.3,panel B)were transfected with pSG-M and pSG-5(vector control).At indicated time-points,cell extracts were prepared and were subjected to western blot analysis using mAb to p53(DO-1)and phospho-p53(serine-15).Data showed that RSV infection increased p53expression and phosphorylation in a time-dependentmanner.Figure 1.RSV-M protein expression inhibits cell proliferation.Panel A,western blot analysis,with specific anti RSV-M monoclonal antibody (C781)in cells transfected with pSG-5plasmid alone (Con)or pSG-5plasmid carrying RSV-M cDNA (pSG-M).Panel B,sub-confluent monolayer (approximately 10%confluency)of A549cells (left panel)and PHBE cells (right panel)were transfected with pSG-M and pSG-5(vector control).Cells were treated with nutlin-3(positive control)and captisol alone (vehicle control).Cell replication was then determined by counting the cell numbers using a hemocytometer.Error bars indicate standard error of the mean (SEM)(n =3).doi:10.1371/journal.pone.0038052.g001Next,we examined p53downstream proteins p21and phospho-Rb.Data showed that p21accumulated significantly starting at 24hr and reached maximal level at 72hr post transfection (Fig.3B and C).The level of Rb phosphorylation was diminished after RSV-M protein transfection,suggesting that downstream signals are involved in cell cycle arrest.The level of RSV-M protein expression was coincident with the maximal effect on changes in the level and the phosphorylation state of the signaling molecules.Quantification of the bands was performed using the ImageJsoftware,and the data showed significant time-dependent changes after RSV-M transfection as compared to vector control (Fig.3C).p53is Necessary for RSV-M Protein Induced Cell Cycle ArrestInfection with several different viruses causes p53activation,which is critical for cell cycle regulation.We next investigated the role of p53in the RSV-M protein induced cell cycle arrest.To this end,a p53-deficient cell line (H1299)was used for transfectionstudies.Figure 2.RSV-M protein induces cell cycle arrest in both A549and PHBE cells.Cells were transfected with pSG-M or pSG-5(vector control).The distribution of cell cycle phases of the transfected A549cells (panel A)and PHBE cells (panel B)were determined by flow cytometry at 2days post-transfection.The cell cycle properties were analyzed using BrdU incorporation and 7-AAD staining.The left panels are FACS data,and the right panels are the statistical analysis of each cycle from 3separate experiments.Error bars are the standard error of the mean (n =3).*p -values ,0.05.doi:10.1371/journal.pone.0038052.g002First,the H1299were transfected with a plasmid carrying the wild-type (wt)p53(pCDNA3.1-p53)or with the vector alone (pCDNA3.1).The stable H1299p53+/+and H1299cell lines were selected with G418.The expression of p53was then assessed by western blot analysis (Fig.4A).Transfections studies were performed with pSG-M and the empty vector.The datafromFigure 3.RSV Infection and RSV-M protein expression induce p53activation.Panel A,A549cells were infected with RSV at MOI of 5PFU/cell.Cell extracts were prepared at indicated times and used in western blot using anti p53mAb (DO-1).A549cells (panel B,left)or PHBE cells (panel B,right)were transfected with pSG-M (M)or pSG-5(V).At indicated time points,total cell extracts were prepared and were used in western blot analysis.The specific anti-total p53(DO-1),anti-phospho-p53at serine-15residue,anti-p21,anti-phospho-Rb at residue 780and anti-RSV-M protein antibody were used to identify the respective molecules.Anti-GAPDH antibody was used as an internal control.Panel C,qualification of p53,phospho-p53,p21,and phospho-Rb was performed by using ImageJ software.Error bars are the standard error of the mean (n =3).*p -values ,0.05.doi:10.1371/journal.pone.0038052.g003cell cycle analysis showed that in H1299expressing of M protein alone did not induce the cell cycle arrest(G1phase:43.065.1% vs.45.963.5%)(Fig.4B).On the other hand,in H1299p53+/+ cells,the pSG-M transfection induced the G1phase arrest(G1 phase:53.3610.3%vs.39.962.7%)(Fig.4C).These results showed that RSV-M protein induced cell cycle arrest is dependent on p53expression.Wild Type p53Expression Enhances RSV Replication Replication of several viruses is enhanced by p53[29,30]. Therefore,we next examined the effect of p53on RSV replication. We tested the RSV transcription and replication in H1299and H1299p53+/+cell lines.First,RSV transcription level was determined by RT-PCR utilizing the RSV-NS1,NS2,N,P,M,G and L gene-specific primers.RT-PCR results showed that at24hr post infection RSV genes mRNA levels are approximately10fold higher in H1299p53+/+cell line than H1299cells(Fig.5A).The enhancement increases to approximately20fold at48hr post infection(Fig.5A).Next,to directly measure the effects of p53 expression on RSV replication,virus yield was determined by plaque assay.Plaque assay results showed that virus titer was approximately20fold higher in H1299p53+/+cell line(Fig.5B). Further,induction of p53in A549and PHBE cells with nutlin-3 significantly enhanced transcription of RSV NS1and G genes. (Fig.5C).The results suggest that the p53protein enhances RSV replication.DiscussionA number of viruses affect the cell cycle to subvert host-cell functions and increase their own replication.The cell cycle arrest during virus infection has adverse effects on lung epithelium and contributes to impede lung repair,which is associated with airways injury and bronchiolitis[31,32].This study for the first time demonstrates that the RSV-M protein causes the epithelial cell cycle arrest by a p53dependent pathway.Modulation of cell growth is a common feature of virus infections and can be induced by specific viral proteins[26,27,33]. The current study demonstrated that the epithelial cells transiently expressing RSV-M protein grew at a slower rate than cells transfected with an empty vector(Fig.1).Effect of RSV-Mprotein Figure4.p53is necessary for RSV-M-induced cell cycle arrest.Panel A,western blot analysis of H1299cells transfected with a plasmid carrying the wild type p53cDNA.After isolation of stable transformants,H1299cells(panel B)and H1299p53+/+cells(panel C)were transfected with pSG-M(RSV-M)and pSG-5(vector).The cycle phase distribution was then analysed by flow cytometry at48hr post transfection.The right panels are the statistical analysis of each cycle from3separate experiments.Error bars are the standard error of the mean(n=3).*p-values,0.05.doi:10.1371/journal.pone.0038052.g004expression was similar to the effect of nutlin-3,a known cell cycle regulator (Fig.1).The effect of RSV-M protein on cell cycle was maximal at day three post transfection which was coincident with the peak of p53accumulation (Fig.3),this suggests that RSV-M protein affects cell cycle through a p53-dependent pathway.Despite the increase in p53expression,there was no significant difference in the number of dead cells between RSV-M protein expressing cells or cells transfected with vector alone,suggesting that the changes in cell numbers is not due to cell death either by necrosis or apoptosis (data not shown).To further determine the effect of p53on RSV-M protein regulation of cell cycle,we used H1299cells which are deficient in p53expression.Transient transfection studies using H1299cells showed that p53was required for the RSV-M protein-induced cell cycle regulation (Fig.4).RSV-M protein is known to be transferred to the nucleus where it can induce cellular transcrip-tional perturbations.Since p53is a cell stress response factor,it is likely that transcriptional perturbation induced by RSV-M protein regulate p53expression.Under non-stressed conditions,p53is a short-lived protein [34].However,when p53is activated by a variety of stress stimuli including DNA damage and virus infections [35],it is rescued from degradation and then translocated into the nucleus.In the nucleus,it can be phosphorylated on serine-15by ataxia ATM (telangiectasia-mutated)or ATR (ATM and Rad3-related)proteins which causes the initiation of a cascade of subsequent post-translational modifications [36].One important p53targets is p21cip1/waf1which is a direct mediator of cell cycle arrest at the G1and G2/M phase.In turn,p21can complex with specific Cdks resulting in inhibition of Rb phosphorylation.The phosphorylation of Rb is a well-described regulator of cell proliferation by controlling progression through the cell cycle restriction points [37].Alteration in Rb phosphorylation is widely used as an indicator of G1/S phase perturbations.In our experiments,results of western blots showed the expression of RSV-M protein induced accumulation of p21and reduction in Rb phosphorylation (Fig.3).The cell cycle analysis on RSV-infected cells or RSV-M protein transfected cells showed differences between A549cellFigure 5.p53expression enhances RSV replication.Panel A,H1299and H1299p53+/+cells were infected with RSV at MOI of 1PFU/cell.After 24and 48hours,total cellular RNA was isolated.The RSV gene transcription levels between the p53-expressing and non-expressing cells were normalized to GAPDH and compared at the respective time points.Panel B,cells were prepared as in panel A but to measure virus replication a standard plaque assay was performed (left panel).The number of plaques and the titer of the virus was then determined (right panel).Panel C,A549and PHBE cells were treated with nutlin-3or captisol alone (vehicle control).After 48hours,cells were infected with RSV at MOI of 1PFU/cell.After 24hr,relative changes of NS1and G genes were determined.The error bars are standard error of the mean from three independent assays.doi:10.1371/journal.pone.0038052.g005line and primary human epithelial cells.In A549cells,the cell cycle was arrested in the G1(Fig.2A),whereas both G1and G2phases were arrested in the PHBE cells (Fig.2B).Our current data obtained in infected A549cells are consistent with the cell cycle arrest by RSV infection that we previously reported [7].However,in PBHE cells,in addition to G2/M arrest RSV-M transfection also induced G1arrest.At this point we do not know the exact reason for this discrepancy but it may be due to more complex events that take place during virus infection as compared to transfection with one protein alone.Also,during transfection,soluble M protein may be present at higher levels as compared to the levels during RSV infections where it can form complexes with other viral proteins [38].Alternatively,it may be due to differences in the nature of each PHBE cell lot,which are isolated from different donors.These differences include but are not limited to sex,age and the genetic backgrounds of each donor.Results of further studies comparing these variables are required to provide clearer answers for the discrpencies in using different lots of PHBE cells.The effect of p53on virus replication has been previously reported.p53protein promotes adenoviral and CMV replica-tion and limits poliovirus and vesicular stomatitis virus replication [29,30,39,40].In this study,we demonstrated that p53expression significantly enhanced the RSV replication,although the exact underling mechanism is still unclear.It is possible that inhibition of the cell cycle results in increased availability of necessary components for viral assembly.It is also possible that p53alone or in addition to other transcription factors act as an enhancing factor for viral replication through a transcriptional effect.It appears that the nuclear shuttling of viral proteins affecting host cell biological activity is an important mechanism to enhance virus replication.Research on nuclear shuttling protein p17of avian reovirus showed that it facilitates virus replication by initiation of p53-dependent G2/M arrest [24,33].In addition,EBV nuclear antigen 3C targets p53and modulates apoptotic activities,and HIV-1nuclear protein Vpr induces cell cycle arrest [41,42,43].Chulu et al.reported that during reovirus infection,the cell cycle blockade promotes virus growth by diverting the cellular machinery required for normal cell replication [24].During HIV-1and CMV infections,cell cycle is arrested at G1or G2/M phase which creates a favorable environment for viral replication [43,44,45].Furthermore,Julia et al.reported that RSV fusion protein triggered p53-dependent apoptosis [25].In conclusion,our data suggests that RSV infections and expression of the M protein profoundly affect cell cycle through a p53-dependent pathway.This suggests that any environmental factor that can increase p53such as air pollution,smoking and ozone could enhance viral replication (Fig.6).In addition,regulation of cell cycle during RSV infection may play a role in damage and recovery during such infections.It is important to note that RSV is the most common virus associated with severe bronchiolitis [46].Further experiments are underway to de-termine the interaction between RSV-M protein and p53regulation on RSV replication and respiratory damage.Author ContributionsConceived and designed the experiments:FI TB.Performed the experiments:FI TB JG CO.Analyzed the data:FI TB JG.Contributed reagents/materials/analysis tools:FI TB JG CO.Wrote the paper:FITB.Figure 6.A working model of the effects of environmental factors on p53expression and enhancement of virus replication.doi:10.1371/journal.pone.0038052.g006。
