Paclitaxel (Taxol) 紫杉醇综述
紫杉醇

紫杉醇针剂
市售成品一般为注射液
性状:无色或淡黄色透明粘稠液体 规格:5ml/30mg 适应症:卵巢癌和乳腺癌,对肺癌、大肠
癌、黑色素瘤、头颈部癌、淋巴瘤、脑瘤 也都有一定疗效。
用法用量:将紫杉醇用5%葡萄糖生理盐
水稀释成0.3~1.2 mg/ml溶液,静滴3小 时。
价格昂贵
国际市场上高纯度紫杉醇(>98%)价格在40万美元/kg以 上。
2. 粉碎
粉碎室内的温度一般不能超过60℃,采用功率和容量尽量大的 粉碎机,粉碎的粒度以40目左右为宜。
3. 95%乙醇浸取
原料+2700L 95%乙醇 浸取24h
残渣
残渣+1600L 95%乙醇 浸取24h
残渣
残渣+1500L 95%乙醇 浸取24h
浸取液
浸取液
作为下一轮第一 次浸取的溶剂
1100kg糖浆状浸膏
cephalomannine
HPLC分析结晶 产物紫杉醇含量 为62.5%
10-DAB
紫杉醇
注意事项:
1. 重结晶过程中加水量和加水速 度都影响结晶效果,应避免溶 液的过饱和度过大。
2. 结晶的母液经氯仿萃取两次, 氯仿萃取液经浓缩、拌样后回 第三次柱层析。
0 5 10 15 20 25 时间/min
4. 液液分配(萃取剂:氯仿)
浸膏
水
氯仿
料液
搅拌30min
含有紫杉醇的 静置2h 氯仿萃取液
液液分配过程中极易发生乳化现象,严重影响成本和产物的收率,所 以这一步骤是分离纯化紫杉醇的难点之一。
应对方法:
1. 浸膏中的乙醇浓度是液液分配的关键。真空浓缩浸取液制备浸膏 过程中调节乙醇含量,使液液分配器中水相的乙醇浓度控制在 10%~15%。
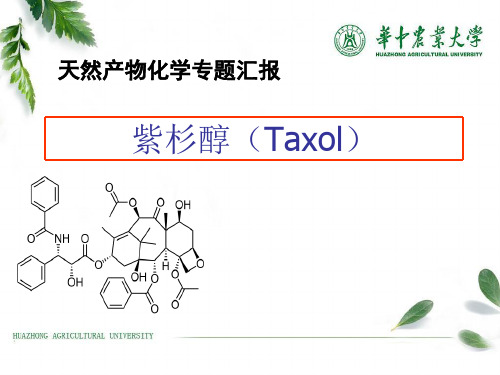
紫杉醇(Taxol)
紫杉醇的化学半合成
有机合成不仅有效地解决了紫杉醇分离提纯难题 ,同时,通过对紫杉醇及其衍生物进行化学修饰 ,使之具有更好的制剂性和生物活性。
美国BMS公司、法国普朗克制药公司等生产紫杉 醇的主要企业现已利用半合成法进行工业化生产 紫杉醇。
紫杉醇的化学全合成
紫杉醇是分子结构比较复杂的手性化合物,现已 完成6条合成路线,总结归纳起来主要有直线法、 会聚法以及这2种方法的联合应用。
利用红豆杉侧芽通过离体培养获取大量的种苗,规模 化、集约化营造原料林
根培养法
利用根癌农杆菌侵染红豆杉的叶片获得发状根,从中 提取紫杉醇
植物细胞培养法
紫杉醇是植物细胞的次生代谢产物,能否通过大 规模的植物细胞培养大量获取紫杉醇具有很高的 研究价值。
许多研究者在高产细胞系的选择和代谢调控方面 进行了大量的卓有成效的研究。
—紫杉醇能结合并稳定微管, 阻止微管的解聚
—导致细胞分裂时不能形成纺 锤体,有丝分裂受阻,细胞周 期停滞于G2/M期
紫杉醇的临床使用
—一线广谱抗癌药
—主要用于治疗乳腺癌,卵巢癌,非小细 胞肺癌和卡波西肉瘤
—聚氧乙基代蓖麻油与无水乙醇的混合溶媒具 有致敏性,直接注射的过敏率高达34.17%
—用药前需对患者注射受体拮抗剂,以降低过 敏反应的发生
目前未能实现工业化生产,这主要是因为:植物 细胞生长慢,紫杉醇含量低,使得单位时间单位 质量细胞紫杉醇含量较低,即使在理论上工业化 生产也难以取得经济效益。
工厂化生产方法
目前,已经应用于大规模工业化生产紫杉 醇的方法主要有两种:
从人工栽培的植物中提取紫杉醇 紫杉醇半合成法
紫杉醇与多烯紫杉醇
紫杉醇的来源
平均每棵树仅能提供 2 kg 左右的树皮,紫杉树矮小, 长 势极为缓慢,1kg干树皮只能提取50~100mg的紫杉醇。 据布一迈施贵宝公司估计, 每例病人的治疗剂量为 150mg/m2, , 对一位体表面积1.6 m2的人, 每个疗程总 剂量为250mg, 每3周为一疗程, 约需3~6个月的疗程,需 要紫杉醇约2g。
taxol

3.2.1.3 不对称氨基羟基化 【7】 反应式如下:
O
DHQ,K2OsO2(OH)4
RHN
O
Ph
OMe
RNNa , H2O
Cl
Ph
OMe
OH
3.2.1.4 Jacobsen 环氧化法【7】
反应式如下:
NH2
O
BzHN
O
Ph
CH2OH a
H
O
H
b
c
Ph
OMe
Ph
OMe
Ph
CO2Me
OH
OH
a : Mn-Salen,4-苯基吡啶-N-氧化物,NaOCl
反应式如下:
N3
O
BzHN
O
Ph
CH2OH a
H
O
H
b
c
Ph
OMe
Ph
OMe
Ph
CO2Me
OH
OH
其中 a:(1)t-BuOOH,TiሺOPr − iሻସ, (+) –DET; (2)RuClଷ , NaIOସ, ,NaHCOଷ; (3)CHଶNଶ;
b:ሺ1ሻ MeଷSiNଷ,ZnClଶ ;(2) HଷOା ; C: (1) Hଶ ,Pd/C ; (2)PhCOCl
3.2 全合成 在 20 世纪 90 年代,出现的 6 中最经典的合成 taxol 方法分别是: 1、霍尔顿(Robert A. Holton)紫杉醇全合成 1994 年 策略:线性合成 AB → C →D 2、尼古劳(Nicolaou)紫杉醇全合成 1994 年 策略:收敛合成 A + C → ABC → D 3、丹尼谢夫斯基(Danishefsky)紫杉醇全合成 1996 年策略:收敛合成 C + D → + A → ABCD 4、温德尔(Wender)紫杉醇全合成 1997 年 策略:线性合成 AB → C → D 5、向山光昭(Kuwajima)紫杉醇全合成 1998 年 策略:线性合成 A → B → C → D 6、桑嶋功(Mukaiyama )紫杉醇全合成 1998 年策略:线性合成 B → C → A →D 这里我们并不详细介绍他们的合成路线和思路。而是通过紫杉醇的结构和逆向成 分析来分解讨论紫杉醇的合成: 紫杉醇的逆向合成分析如下:
抗癌药紫杉醇

天然药物史话——紫杉醇【摘要】紫杉醇是从红豆杉的树皮,树根及其枝叶中提取的一种化合物,于1992年底被FDA批准作为抗晚期癌症新药上市。
美国肿瘤研究所认为紫杉醇是人类未来20年间最有效的抗癌药物之一。
紫杉醇类药物具有良好的抗肿瘤活性与其独特的抗肿瘤机理,研究表明紫杉醇可能通过多种信号传导通路和机制共同参与细胞凋亡。
尽管紫杉醇和多烯紫杉醇临床治疗上均有突出的贡献,但是依然存在诸多临床缺陷,例如水溶性差,耐药性以及毒性反应。
其中毒性反应包括骨髓抑制,神经毒性,肌肉关节疼痛,心脏毒性等。
近年以来对紫杉醇类药物进行水溶性的改善有很多报导。
一方面,通过前药策略对其结构进行化学修饰。
引入极性较大的基团用以提高化合物的水溶性。
包括引入氨基酸类,糖类,有机酸类水溶性片段以及成盐等,然而大多数却停步于临床药物阶段。
另一方面,通过不同的剂型进行研究也是改善其水溶性的另一种手段与策略,包括乳剂,脂质体,聚合物胶束和纳米粒等。
不过按其推荐给药方案计算费用约为进口普通紫杉醇的两倍,限制了其临床应用。
因此对紫杉醇类药物改善依旧需要进一步探索和研究,以期待更加安全有效经济的治疗药物。
【关键词】抗肿瘤药物、紫杉醇、紫杉醇作用机制、紫杉醇水溶性、紫杉醇类药物毒副作用、前药设计一.紫杉醇与癌症根据卫生部统计,近年来我国每年新增肿瘤患者212. 7万人,其中每年有106万左右的恶性肿瘤新生患者;全国约有268. 5万左右的肿瘤现有患者,其中,恶性肿瘤现有患者约148. 5万左右;肿瘤死亡率呈现明显上升趋势,兼有发展中国家和发达国家高发谱并存的特点。
1从全球情况来看,肿瘤发生率预计在2000 - 2020年间将上升。
2随着科学,医疗诊断技术的进步,癌症已经人类致死的第一大死因,但是医学的进步和新药的研究上市,给人类战胜癌症带来了曙光。
同时,正因为全球癌症发病率的持续上升,抗肿瘤药物的市场的不断扩大,也为抗肿瘤药物的研发提供了巨大的机遇和挑战。
简述紫杉醇的药理学知识

简述紫杉醇的药理学知识紫杉醇(Paclitaxel),又称紫杉素,是一种从西夏树叶和树皮提取的天然二萜类化合物。
它是一种非常重要的抗肿瘤药物,广泛应用于治疗多种恶性肿瘤,特别是乳腺癌、卵巢癌、非小细胞肺癌和胃肠道肿瘤等。
紫杉醇通过与微管结合,干扰肿瘤细胞有丝分裂过程,从而抑制肿瘤的生长和扩散。
紫杉醇的药理学机制主要涉及以下几个方面:1.微管动力学抑制:紫杉醇是一种微管抑制剂,它通过与微管结合,干扰正常微管的正常动态重组过程。
正常的微管动力学是在细胞内维持细胞形态、细胞内运输和细胞有丝分裂等过程中起到重要作用的关键因素。
紫杉醇的结合导致微管的稳定以及抑制微管动力学,阻断了肿瘤细胞的有丝分裂过程,从而阻止细胞增殖。
2.细胞周期阻滞:紫杉醇也可以阻滞细胞周期的进程,抑制肿瘤细胞的增殖。
它主要通过调控细胞周期蛋白和细胞周期细胞因子的表达来实现。
紫杉醇对细胞周期的影响主要涉及到G2/M期的停滞,即在细胞准备进入有丝分裂的G2期时对细胞进行阻滞,从而阻碍细胞分裂和增殖。
3.细胞凋亡诱导:与微管动力学抑制和细胞周期阻滞相比,细胞凋亡诱导是紫杉醇对肿瘤细胞的另一个主要作用机制。
紫杉醇通过激活与细胞凋亡相关的蛋白激酶Caspase,从而诱导肿瘤细胞的凋亡。
此外,紫杉醇还通过调控Bcl-2家族蛋白和细胞凋亡相关基因的表达来促进细胞凋亡的发生。
4.血管生成抑制:血管生成是肿瘤生长和转移的关键过程,紫杉醇在抑制肿瘤的血供和血管生成上也具有重要作用。
它通过抑制血管内皮细胞的增殖和迁移,以及下调相关血管生成因子(如VEGF)的表达来实现抗血管生成的效果。
5.免疫调节:最近的研究表明,紫杉醇还可以调节机体的免疫应答,增强机体对肿瘤的免疫监视。
这一机制主要通过抑制肿瘤相关的免疫抑制因子(如Treg细胞)的产生和功能,以及增强T细胞的活化和杀伤功能。
总体而言,紫杉醇是一种作用于多个靶点的抗肿瘤药物,通过微管动力学抑制、细胞周期阻滞、细胞凋亡诱导、血管生成抑制和免疫调节等多种机制来抑制肿瘤的生长和扩散。
紫杉醇(TAX、泰素、特素、紫素)

【药物名称】中文通用名称:紫杉醇英文通用名称:Paclitaxel其它名称:安素泰、泰素、特素、紫素、Anzatax、Paclitaxelum、PTX、Taxol【临床应用】主要用于治疗卵巢癌和乳腺癌(经一线化疗或多次化疗失败的卵巢癌;经联合化疗失败的转移性乳腺癌或经辅助性化疗后6个月内复发的乳腺癌),对肺癌、食管癌、胃癌、软组织肉瘤、大肠癌、黑色素瘤、头颈部癌、淋巴瘤、脑瘤、精原细胞瘤等也有一定疗效。
【药理】1.药效学本药是从短叶紫杉(Taxus brevis)中提取或半合成的一种抗癌药。
可作用于细胞微管/微管蛋白系统,促进微管蛋白装配成微管,但同时抑制微管的解聚,从而导致微管束的排列异常,形成星状体,使纺锤体失去正常功能,从而导致细胞死亡。
本药还可在缺少鸟苷三磷酸(GTP)与微管相关蛋白(MAP)的条件下,诱导形成无功能的微管。
在体外人瘤株筛选和实验动物中本药对多种肿瘤均有效,属于广谱抗肿瘤药。
对顺铂、多柔比星耐药者,使用本药也有效。
2.药动学本药静脉滴注后,血药峰值浓度(Cmax)为435-802ng/mL,滴注结束6-12小时后,血药浓度仍可达具有细胞毒活性的水平(85ng/mL)。
血浆蛋白结合率为89%-98%。
在血浆内消除呈二室模型,平均半衰期α相为0.27小时,β相为6.4小时。
主要在肝脏代谢,经胆汁随粪便排泄,仅有少量(约占给药量的13%)以原形从尿中排出。
【注意事项】1.禁忌症 (1)对本药过敏者。
(2)孕妇及哺乳妇女。
(3)白细胞计数低于1.5×10×E9/L 的严重骨髓抑制者。
(4)中性粒细胞计数低于1×10×E9/L的艾滋病相关性卡波西肉瘤(Kaposi's肉瘤)患者(国外资料)。
2.慎用 (1)有心脏传导功能异常者。
(2)低血压或心动过缓者(国外资料)。
(3)有周围神经病变者(国外资料)。
3.用药前后及用药时应当检查或监测用药期间应定期检查白细胞及血小板计数、肝肾功能、心电图等。
紫杉醇(Paclitaxel):一种广泛应用的抗癌化疗药物的详细介绍

紫杉醇(Paclitaxel):一种广泛应用的抗癌化疗药物的详细介绍
摘要:本文将详细介绍紫杉醇(Paclitaxel),这是一种广泛应用的抗癌化疗药物。
紫杉醇属于植物生物碱类,具有强大的抗肿瘤活性,可用于乳腺癌、卵巢癌和非小细胞肺癌等多种癌症的治疗。
我们将探讨紫杉醇的作用机制、药理特点、临床应用、副作用以及注意事项。
1. 作用机制:
-紫杉醇通过干扰微管的动态稳定性,阻止癌细胞的正常有丝分裂过程。
-它结合并稳定微管,阻碍肿瘤细胞的分裂和扩散,导致细胞凋亡和生长抑制。
2. 药理特点:
-紫杉醇可以通过静脉输注给药,进入血液循环,并迅速分布到全身各组织器官与肿瘤部位。
-它主要在肝脏代谢,并通过胆汁和粪便排泄。
3. 临床应用:
-紫杉醇广泛应用于多种癌症的治疗,包括但不限于:
-乳腺癌、卵巢癌和子宫内膜癌等生殖系统恶性肿瘤;
-非小细胞肺癌、胃癌和食管癌等其他类型的恶性肿瘤。
4. 副作用:
-紫杉醇的常见副作用包括恶心、呕吐、脱发、周围神经病变、骨髓抑制等。
-稀有但严重的副作用可能包括过敏反应、感染、心脏毒性和神经毒性等。
5. 注意事项:
-使用紫杉醇需在医疗监督下进行,并需遵循相关的用药指导和剂量调整。
-患者在治疗期间需要定期进行血常规、肝功能和神经功能监测。
-在使用紫杉醇期间,患者需告知医生关于任何新出现的不适或副作用。
紫杉醇(TAX、泰素、特素、紫素)

【不良反应】
1.血液系统 骨髓抑制是本药主要的剂量限制性毒性。常见中性粒细胞减少,最低值一般在给药后第11日出现,通常停药后能很快恢复。偶见血小板减少和血红蛋白下降,与给药的次数和总量有关。
6.静脉滴注本药的最初1小时内,应每15分钟测血压、心率和呼吸1次,并注意观察有无过敏反应。
7.国外资料提示,开始新的疗程须具备以下条件:中性粒细胞计数至少为1.5×10×E9/L、血小板计数至少为100×10×E9/L。如果患者出现严重的中性粒细胞减少(计数低于0.5×10×E9/%。
在体外人瘤株筛选和实验动物中本药对多种肿瘤均有效,属于广谱抗肿瘤药。对顺铂、多柔比星耐药者,使用本药也有效。
2.药动学 本药静脉滴注后,血药峰值浓度(Cmax)为435-802ng/mL,滴注结束6-12小时后,血药浓度仍可达具有细胞毒活性的水平(85ng/mL)。血浆蛋白结合率为89%-98%。在血浆内消除呈二室模型,平均半衰期α相为0.27小时,β相为6.4小时。主要在肝脏代谢,经胆汁随粪便排泄,仅有少量(约占给药量的13%)以原形从尿中排出。
[国外用法用量参考]
成人
·常规剂量
·静脉滴注
1.乳腺癌:(1)转移性乳腺癌,联合化疗失败或者经辅助化疗6个月内复发的转移性乳腺癌患者,建议每次175mg/m2,滴注时间至少3小时,每3周1次。(2)淋巴结阳性的乳腺癌患者,进行包括多柔比星在内的标准联合化疗方案,本药每次175mg/m2,滴注时间至少3小时,每3周1次。连用4个疗程。
3.顺铂可使本药的清除率降低约1/3,若先给顺铂再给予本药,可产生更为严重的骨髓抑制。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1004 THE NEW ENGLAND JOURNAL OF MEDICINE April 13, 1995REVIEW ARTICLEDRUG THERAPYA LASTAIR J.J. W OOD , M.D., EditorPACLITAXEL (TAXOL) E RIC K. R OWINSKY , M.D., AND R OSS C. D ONEHOWER , M.D.From the Divisions of Pharmacology and Experimental Therapeutics (E.K.R.)and Medical Oncology (R.C.D.), Oncology Center, Johns Hopkins University School of Medicine, Baltimore. Address reprint requests to Dr. Rowinsky at the Johns Hopkins Oncology Center, Division of Pharmacology and Experimental Therapeutics, 1-121, 600 N. Wolfe St., Baltimore, MD 21287-8934.THE taxanes are an important new class of anti-cancer agents that exert their cytotoxic effects through a unique mechanism. Paclitaxel (Taxol), the first taxane in clinical trials, is active against a broad range of cancers that are generally considered to be re-fractory to conventional chemotherapy. This has led to the regulatory approval of paclitaxel in the United States and many other countries for use in the pallia-tive therapy of patients with ovarian and breast cancers resistant to chemotherapy. The challenge now is to de-velop strategies using paclitaxel in the initial therapy of cancers in which cure or improved survival may be an achievable goal.Paclitaxel was discovered as part of a National Can-cer Institute program in which extracts of thousands of plants were screened for anticancer activity. In 1963, a crude extract from the bark of the Pacific yew T axus brevifolia, a scarce and slow-growing evergreen found in the old-growth forests of the Pacific Northwest, was found in preclinical studies to have cytotoxic activity against many tumors. 1 Paclitaxel was identified as the active constituent of this extract in 1971. 1 Although it had a novel chemical structure (Fig. 1) and broad pre-clinical activity, development was slowed because it did not appear to be more effective against experimental tumors than other agents under development at that time. In addition, it was expected that the procurement and preparation of this potentially scarce natural prod-uct in sufficient quantities for large-scale development would be arduous. Interest was revived in 1979 when paclitaxel’s unique mechanism of action as an antitu-mor drug was identified, and was further stimulated when impressive activity was demonstrated in the Na-tional Cancer Institute tumor screening. 2-4M ECHANISMS OF A CTION AND R ESISTANCEMicrotubules are composed of polymers of tubulin in dynamic equilibrium with tubulin heterodimers com-posed of alpha and beta protein subunits. 4-6 Although their principal function is the formation of the mitotic spindle during cell division, microtubules are also in-volved in many vital interphase functions, including the maintenance of shape, motility, signal transmis-sion, and intracellular transport. 4-7 Unlike other an-timicrotubule drugs, such as vinca alkaloids, which induce the disassembly of microtubules, paclitaxel pro-motes the polymerization of tubulin. 2,3,8-14 At subnano-molar concentrations, paclitaxel inhibits the disassem-bly of microtubules, whereas it increases their mass and numbers at higher, albeit clinically achievable,concentrations. 14 The microtubules formed in the pres-ence of paclitaxel are extraordinarily stable and dys-functional, thereby causing the death of the cell by dis-rupting the normal microtubule dynamics required for cell division and vital interphase processes. Paclitaxel also induces the expression of the gene for tumor ne-crosis factor a , but structure–activity studies indicate that these activities are not related to paclitaxel’s ef-fects on microtubule assembly, raising the issue of what part these cytokines play in the antitumor activity of paclitaxel. 15 The binding site for paclitaxel is dis-tinct from the binding sites for guanosine triphosphate,colchicine, vinblastine, and podofilox (podophyllotox-in). 4,8-11,15 Paclitaxel binds to the N-terminal 31 amino acids of the beta-tubulin subunit in the microtubule,rather than to tubulin dimers. 8,9,16,17 In intact cells, pac-litaxel induces the bundling of microtubules, which may be a useful clinical correlate of a lethal drug ef-fect, 3,4,18-20 and the formation of large numbers of asters of mitotic spindles (Fig. 2). 4,18-21 It also enhances the cytotoxic effects of ionizing radiation in vitro, possibly by inducing arrest in the premitotic G 2 and mitotic phases of the cell cycle, which are the most radiosensi-tive phases. 22,23 The feasibility of using paclitaxel in combination with radiation to treat patients with local-ly advanced lung, head and neck, and esophageal can-cers, which are responsive to both kinds of treatment,is currently being evaluated. 24Two mechanisms of acquired resistance to the tax-anes have been characterized. First, some tumors con-tain alpha- and beta-tubulin with an impaired ability to polymerize into microtubules and have an inherent-ly slow rate of microtubule assembly that is normal-ized by the taxanes. 25 A second mechanism involves the amplification of membrane phosphoglycoproteins that function as drug-efflux pumps. 26,27 The multidrug-resistant phenotype of tumor cells confers varying de-grees of cross-resistance to various structurally bulky natural products, including anthracyclines, etoposide,vinca alkaloids, colchicine, and taxanes. The contribu-tion of these mechanisms to clinical drug resistance is not known, but the results of early clinical studies of patients with breast cancer suggest a lack of completeVol.332No.15DRUG THERAPY 1005cross-resistance between the taxanes and anthracy-clines that would not be expected if the multidrug-resistant phenotype was an important mechanism of resistance.T OXICITYIn the early phase 1 trials, a number of obstacles,particularly hypersensitivity reactions, were encoun-tered that threatened the prospects for paclitaxel’s fur-ther development. Table 1 shows the results of phase 1trials of paclitaxel as a single agent that have been performed in the United States. 28-40 Neutropenia was the principal toxic effect, but several others were en-countered, along with unique pharmaceutical proper-ties. 4,41,42Hypersensitivity ReactionsA difficult problem encountered during the early de-velopment of paclitaxel was the high incidence of ma-jor hypersensitivity reactions, approaching 25 to 30percent in some studies. M ost affected patients had type 1 hypersensitivity reactions, including dyspnea with bronchospasm, urticaria, and hypotension. 43 Seri-ous reactions usually occurred within 2 to 3 minutes after the administration of paclitaxel, and almost all occurred within the first 10 minutes. The majority oc-curred after the first or second dose. One fatality was reported; all other patients recovered fully after the discontinuation of paclitaxel and with occasional treat-ments with antihistamines, fluids, and vasopressors.Although flushing and rashes have also been noted in as much as 40 percent of patients, minor reactions do not portend the development of major ones. 44,45Initial observations suggested that these hypersensi-tivity reactions were mediated by the direct release ofhistamine or other vasoactive substances, as are the hy-persensitivity reactions caused by radiographic contrast agents. 4,41,43 Although these reactions could have been caused by paclitaxel itself or its polyoxyethylated castor oil vehicle (Cremophor EL), the latter was thought to be responsible, since it induced histamine release and similar manifestations in dogs 46 and since other drugs formulated in polyoxyethylated castor oil, such as cyclo-sporine and vitamin K, have been associated with sim-ilar reactions. 44,47 The phase 1 trials were completed with the use of 24-hour infusions and premedication with corticosteroids and histamine H 1 and H 2 antago-nists, since similar regimens have proved effective in preventing repeated reactions to radiographic contrast agents. This schedule has been used in the majority of phase 2 and 3 studies, as well. The following premedi-cation is currently recommended: 20 mg of dexameth-asone orally or intravenously 12 and 6 hours before treatment; 50 mg of diphenhydramine intravenously 30minutes before treatment; and a histamine H 2 antago-nist such as cimetidine (300 mg), famotidine (20 mg),or ranitidine (150 mg) intravenously 30 minutes before treatment. Although these measures are not fully pro-tective, the incidence of major hypersensitivity reac-tions has decreased to approximately 1 to 3 percent. 41,44 The National Cancer Institute of Canada Clinical Tri-als Group evaluated the relative safety and efficacy of two paclitaxel doses (135 and 175 mg per square meter of body-surface area) and two infusion schedules (for 24 hours and for 3 hours) with standard premedication in women with recurrent or refractory ovarian cancer. 45 The overall incidence of major hypersensitivity reac-tions was similar in women receiving paclitaxel for 3 hours (2.1 percent) and in those receiving it for 24hours (1.0 percent), with premedication. These findings will have a substantial impact if the antitumor activity of the 3-hour and 24-hour infusions is equivalent, as initially reported in these patients, since shorter infu-sions are more convenient and less expensive. Patients with major hypersensitivity reactions who have been re-challenged with paclitaxel after receiving high doses of corticosteroids have not had recurrences, although this approach has not been universally successful. 48,49Hematologic ToxicityNeutropenia is the principal toxic effect of paclitax-el. 41Its onset is usually on day 8 to 10 after treatment,and recovery is usually complete by day 15 to 21. Neu-tropenia is not cumulative, suggesting that paclitaxel does not irreversibly damage immature hematopoietic cells. At doses of 200 to 250 mg of paclitaxel per square meter given over a period of 24 hours, neutro-penia is usually severe even in previously untreated pa-tients, with neutrophil counts decreasing to below 500per cubic millimeter after the majority of infusions.This dose range was initially recommended for phase 2 studies because the duration of severe neutropenia ( Ͻ 500 per cubic millimeter) was usually short ( Ͻ 5Figure 1. Structure of Paclitaxel.OCOCH 3OOHOOCOCH 3H OC OHOO CO 5ЈC N HOHC2ЈCHOH121311109871006THE NEW ENGLAND JOURNAL OF MEDICINE April 13, 1995days) and treatment delays for unresolved toxic effectswere rare. Although the frequency of febrile and infec-tious sequelae at these doses was originally reported tobe lower (Ͻ10 percent of courses) than that of severe neutropenia,50,51 these complications occurred morefrequently in later studies. Therefore, granulocyte colo-ny-stimulating factor is commonly given to prevent thecomplications of neutropenia in trials of doses in thisrange. In most patients, particularly those who have re-ceived large doses of other chemotherapeutic agentspreviously, the maximal tolerated dose without granu-locyte colony-stimulating factor is 175 to 200 mg persquare meter. The most critical pharmacologic deter-minant of the severity of neutropenia seems to be thelength of time that plasma drug concentrations arehigher than biologically active concentrations (0.05 to0.1 m mol per liter) — a fact that may explain why neu-tropenia is more severe with longer infusions.45,52 Thisdoes not imply that shorter infusions should be used inall patients, since the optimal dose and schedule havenot been determined for most tumors. Notwithstandingthese differences, the principal clinical determinant ofthe severity of neutropenia is the extent of previous my-elotoxic therapy. Paclitaxel alone rarely causes severethrombocytopenia and anemia.NeurotoxicityPaclitaxel induces a peripheral neuropathy that ischaracterized by sensory symptoms such as numbnessand paresthesia in a glove-and-stocking distribution.41,53There is often symmetric distal loss of sensation carried by both large fibers (proprioception, vibration) andsmall ones (temperature, pinprick). Symptoms may be-gin as soon as 24 to 72 hours after treatment with high-er doses (Ͼ250 mg per square meter) but usually occur only after multiple courses at conventional doses (135 to250 mg per square meter). Severe neurotoxicity pre-cludes the administration of paclitaxel doses above 250mg per square meter over a period of 3 or 24 hours, butsevere neurotoxicity is rare at conventional doses (Ͻ200 mg per square meter), even in patients who have previ-ously received other neurotoxic agents, such as cispla-tin. For example, although mild-to-moderate neurotox-icity was reported in 0 to 88 percent of women with recurrent ovarian cancer after receiving paclitaxel in doses of up to 175 mg per square meter, severe toxic ef-fects occurred in only 0 to 3 percent despite prior ther-apy with cisplatin in the majority.50,54,55 The distal, sym-metric, length-dependent neurologic deficits suggest that paclitaxel causes a sensory and motor axonal loss similar to the “dying-back” neuropathies that may have their origin in the cell body or in axonal transport, but a few patients have the simultaneous onset of symptoms in the arms and legs, involvement of the face (perioral numbness), the predominance of large-fiber loss, and diffuse areflexia suggestive of a neuronopathy. Both types of neuropathy depend on the dose of paclitaxel or its combination with cisplatin.53,56 Motor and autonomic dysfunction may also occur, especially at high doses and in patients with preexisting neuropathies caused by dia-betes mellitus and alcoholism. In addition, optic-nerve disturbances, characterized by scintillating scotomata,Figure 2. Microtubule Effects of Paclitaxel in Human Leukemia Cells Stained with Antitubulin Antibody and Viewed by Indirect Immu-nofluorescence Microscopy (ϫ3780).Panel A shows untreated K562 cells; Panel B, microtubule bundles in HL-60 promyelocytic leukemia cells treated with paclitaxel; and Panel C, multiple asters of mitotic spindles in K562 cells treated with paclitaxel.18A B CVol.332No.15DRUG THERAPY1007may occur. 57 Transient myalgia, usually noted two to five days after therapy, is also common at doses above 170 mg per square meter, and myopathy has been noted with high doses of paclitaxel ( Ͼ 250 mg per square meter) in combination with cisplatin. 56,58Cardiac EffectsPaclitaxel causes disturbances in cardiac rhythm,but the importance of these effects is not known. 59-61 The most common effect, a transient asymptomatic bradycardia, was noted in 29 percent of patients in one trial. 50 Isolated asymptomatic bradycardia without he-modynamic effects is not an indication for discon-tinuing paclitaxel. More important bradyarrhythmias,including M obitz type I (Wenckebach’s syndrome),Mobitz type II, and third-degree heart block, have also been noted, 50,59-61 but the incidence in a large National Cancer Institute data base was only 0.1 percent. 61 All events occurred in patients enrolled in trials that re-quired continuous cardiac monitoring, indicating that second- and third-degree heart block is probably un-derreported, since continuous cardiac monitoring is not usually performed. M ost documented episodes have been asymptomatic and reversible. These bradyar-rhythmias are probably caused by paclitaxel, since re-lated taxanes affect cardiac automaticity and conduc-tion and since similar disturbances have occurred in humans and animals that had ingested various species of yew plants. 61M yocardial infarction, cardiac ischemia, atrial ar-rhythmias, and ventricular tachycardia have also beennoted. 50,59-61 Whether there is a direct causal relation between paclitaxel and ventricular and atrial tachycar-dias, or between paclitaxel and ischemic events, is un-certain. 61 There is also no evidence of cumulative tox-icity or augmentation of the acute cardiac effects of the anthracyclines 61 ; however, the frequency of congestive cardiotoxicity in patients treated with paclitaxel and doxorubicin in one trial was higher than expected from the latter alone. 62 In patients treated with paclitaxel and an anthracycline, potential drug effects on ventric-ular function should be evaluated at lower cumulative anthracycline doses than might otherwise be done with an anthracycline alone.Once cardiac effects were documented, eligibility in most trials was restricted to patients with no history of cardiac disease. However, this broadly described popu-lation undoubtedly excludes many patients who might otherwise be good candidates for paclitaxel, and the risk of cardiotoxicity in patients with cardiac disease is not known. Cardiac monitoring during paclitaxel therapy is not necessary routinely but is advisable for patients who may not be able to tolerate the drug’s potential bradyarrhythmic effects, such as those with atrioventricular conduction defects or ventricular dys-function.Miscellaneous Toxic EffectsDrug-related gastrointestinal effects of paclitaxel use,such as vomiting and diarrhea, are infrequent. 41 Higher doses may cause mucositis, especially in patients with leukemia who may be more prone to breakdown of the*G-CSF denotes granulocyte colony-stimulating factor, the doses of which are indicated per kilogram of body weight.†Courses were repeated every 21 days except in the study by Rowinsky et al., 20 in which courses were repeated every 14 to 21 days. Treatment was continued until the disease progressed, serious toxic reactions occurred, or the patient withdrew from the study.‡This was the highest dose given in studies that were terminated because of major hypersensitivity reactions.§The duration of the infusion was lengthened during the study, and premedication was added because of major hypersensitivity reactions.¶The patients were children with refractory solid tumors. ʈ The patients had refractory leukemia.**The patients had advanced-stage ovarian cancer that was recurrent or refractory to treatment with platinum.††The patients had metastatic breast cancer and lymphoma and had previously received large doses of other chemotherapeutic agents.Table 1. Phase 1 Studies of Paclitaxel in the United States. *S TUDYD URATION OF I NFUSION(P REMEDICATION )†R ECOMMENDED P HASE 2 D OSE D OSE -L IMITING T OXIC E FFECTO THER T OXIC E FFECTSLegha et al. 28 1 hr ϫ 5 days (no)40 mg/m 2 ϫ 5Neutropenia Alopecia, diarrheaGrem et al. 29 1–6 hr ϫ 5 days (no)30 mg/m 2 ϫ 5NeutropeniaHypersensitivity reactions, nausea, vomiting, mu-cositis, thrombocytopeniaKris et al. 303 hr (no)190 mg/m 2 ‡Hypersensitivity reactions Neutropenia, nausea Schiller et al. 31 3 hr (yes)210 mg/m 2Neutropenia Neurotoxicity Schiller et al. 31 3 hr ϩ G-CSF (yes)250 mg/m 2 ϩ 5 m g of G-CSF/kg/day Neurotoxicity —Donehower et al. 32 1–6 hr (yes)§210 mg/m 2 Neutropenia Neuropathy, mucositis, myalgia, hypersensitivityreactionsBrown et al. 33 6 hr (no)225 mg/m 2NeutropeniaMyalgia, neuropathy, mucositis, hypersensitivity reactionsWiernik et al. 34 1–6 hr (yes)§250 mg/m 2 Neutropenia Mucositis, neuropathy, hypersensitivity reactions Wiernik et al. 35 24 hr (yes)250 mg/m 2 Neutropenia Hypersensitivity reactions, neuropathy Ohnuma et al. 36 24 hr (no)200 mg/m 2 ¶Neutropenia Nausea, vomitingHurwitz et al. 37 24 hr (yes)¶350 mg/m 2 Neurotoxicity Neutropenia, mucositis, thrombocytopeniaRowinsky et al. 20 24 hr (yes) ʈ 310 mg/m 2Mucositis Neutropenia, neuropathy, hypersensitivity reactions Sarosy et al. 3824 hr ϩ G-CSF (yes)**250 mg/m 2 ϩ 10 m g of G-CSF/kg/day NeuropathyNeutropenia, cardiac toxicity, thrombocytopenia, myalgiaWilson et al. 3996 hr (no)††140 mg/m 2 Mucositis, neutropenia —Spriggs and Tondini 40120 hr (no)150 mg/m 2NeutropeniaMucositis1008THE NEW ENGLAND JOURNAL OF MEDICINE April 13, 1995mucosal barrier 20,37 or in patients receiving 96-hour in-fusions. 41 Rare cases of neutropenic enterocolitis have occurred, particularly in patients given high doses of paclitaxel in combination with doxorubicin or cyclo-phosphamide. 63,64 Like other chemotherapeutic agents,paclitaxel induces reversible alopecia of the scalp, and all body hair is often lost with cumulative therapy. In-flammation at the injection site, along the course of an injected vein, and in areas of drug extravasation may occur rarely, as may inflammatory skin reactions over previously radiated sites. 41,65,66C LINICAL P HARMACOLOGYPharmacokinetic data for paclitaxel are shown in Ta-ble 2. The clearance of the drug appeared to be linear in early studies of prolonged infusions,33-35,67,69 but clearance may be nonlinear or saturable when the drug is infused for shorter periods, with both peak plasma concentrations and drug exposure increasing dispro-portionately with increasing doses.58,68-70 The peak plas-ma concentrations in patients assigned to all regimens have been in the range capable of inducing relevant bi-ologic effects in vitro. Despite extensive binding to plas-ma proteins (95 to 98 percent), paclitaxel is readily cleared from plasma. The volume of distribution is large, suggesting binding to cellular proteins, possibly tubulin. Renal clearance accounts for a small propor-tion (1 to 8 percent) of total clearance, and therefore dose modifications do not appear to be necessary in pa-tients with renal dysfunction.33-35,67,69,71 Hepatic metab-olism, biliary excretion, fecal elimination, or extensive tissue binding appears to be responsible for most of the systemic clearance.69,72,73 The biliary concentrations of both paclitaxel and its hydroxylated metabolites,formed by cytochrome P-450 enzyme systems, are high.72-75 The optimal dose for patients with hepatic dysfunction has not been determined, nor has the po-tential for interactions with drugs that may modulate the activity of hepatic P-450 enzymes. On the basis of available data, the doses of paclitaxel should be re-duced by at least 50 percent in patients with moderate or severe hyperbilirubinemia or substantially increased serum aminotransferase concentrations.76As part of the effort to combine paclitaxel with cis-platin after prominent single-agent activity was noted in women with advanced ovarian cancer, the possibility of sequence-dependent drug interactions was studied.59The principal toxic effect, neutropenia, was more se-vere when cisplatin was administered before paclitaxel.This appeared to be due to decreased plasma clearance of paclitaxel after cisplatin, possibly caused by the modulating effects of cisplatin on cytochrome P-450 en-zymes.73,77 The less toxic sequence — paclitaxel fol-lowed by cisplatin — was more cytotoxic to tumor cells in vitro.78 These findings formed the rationale for using paclitaxel followed by cisplatin in trials of combination therapy.79 Important sequence-dependent interactions have also been identified in studies of paclitaxel–doxo-rubicin and paclitaxel–cyclophosphamide regimens.64,80A NTITUMOR A CTIVITYOvarian CancerPaclitaxel was initially approved by the Food and Drug Administration in 1992 for treating women with epithelial ovarian cancer on the basis of the results of trials of 24-hour infusions of paclitaxel alone (Table 3).38,50,54,81,82 These results provided the impetus for broad phase 2 testing. In the first study, 30 percent of a group of heavily pretreated women had major antitu-mor responses. A major response was defined as either a complete response (the complete disappearance of all disease, with normalization of tumor markers) or a par-tial response (a reduction in the sum of the products of the bidimensional measurements of all known sites of disease by at least 50 percent).50 The durations of re-sponse ranged from 1 to 15 months, with a median of 6 months. Twenty-four percent of the women consid-ered resistant to platinum-based therapy (those who had disease progression within six months) responded,whereas 40 percent of those who relapsed more than*Mean values are given. CL denotes systemic clearance, VD ss volume of distribution at steady state, and C peak peak plasma concentration.†Values are taken from a pediatric study demonstrating saturable pharmacokinetics. Clearance values listed are for doses below 400 mg/m 2 (161 ml/min/m 2) and doses above 400 mg/m 2 (123 ml/min/m 2).Table 2. Pharmacokinetic Properties of Paclitaxel.*D URATIONOF I NFUSIONM ODELH ALF -LIFECL VD ss C peak S TUDYALPHABETAGAMMAhours ml/min/m 2liter/m 2m mol/liter (dose)3 hr Triphasic Saturable 0.200.27 1.42.314.418.82942129899 2.5 (135 mg/m 2)4.3 (175 mg/m 2)Huizing et al.526 hr Biphasic0.36 6.4—19559 2.2–13.0 (170–275 mg/m 2)Brown et al.,33 Wiernik et al.,34Longnecker et al.6724 hr Biphasic 0.39 3.3—3591190.6–0.9 (200–275 mg/m 2)Wiernik et al.3524 hr Triphasic Saturable0.090.142.22.049.823.63633936572690.2 (135 mg/m 2)0.4 (175 mg/m 2)Huizing et al.5224 hr Saturable elimination and distribution161†123†(Ͻ400 mg/m 2)(Ͼ400 mg/m 2)Sonnichsen et al.6824 hr 0.2–3.4 (110–390 mg/m 2)Rowinsky et al.2096 hr4370.05–0.08 (120–160 mg/m 2)Wilson et al.39Vol.332No.15DRUG THERAPY 1009six months after receiving platinum therapy (and whomight have responded to repeated platinum therapy)responded. The doses of paclitaxel (110 to 135 mg persquare meter) were substantially lower than those thatcan be safely given to patients who have previously re-ceived less extensive therapy. Although severe neutro-penia occurred during most courses, even at relative-ly low doses, it was short-lived and rarely associatedwith fever. The results of confirmatory trials were sim-ilar.54,81These results were substantially better thanthose of other salvage chemotherapies and comparableto the early results with cisplatin.83.84On the basis of encouraging results with relatively low doses of paclitaxel in women with advanced dis-ease, the potential of granulocyte colony-stimulating factor to permit dose escalation was evaluated.38,82Ma-jor antitumor responses occurred in 48 percent of heavily pretreated patients. The median survival time was 11.5 months, similar to that reported in previous trials using lower doses. The median relapse-free sur-vival time was 6.2 months, and the relapse-free survival rate was 41 percent at 9 months. These results with higher doses suggested the possibility of a relation be-tween the dose of paclitaxel and the response.The generalizability of the results of clinical trials to the treatment of women with advanced ovarian cancer in general oncology practice was demonstrated in a treatment-referral-center program instituted by the National Cancer Institute.55Through this program,paclitaxel (135 mg per square meter over a period of 24 hours) was initially provided to women whose ovarian cancers had progressed after treatment with three regimens. Twenty-two percentof the first 1000 patients had major responses despite their poor prog-nostic characteristics.With the demonstration that pac-litaxel and cisplatin could be safelycombined,59 a next logical step was to compare paclitaxel (135 mg per square meter) followed by cisplatin (75 mg per square meter) with a standard regimen of cyclophospha-mide (750 mg per square meter)and cisplatin (75 mg per square meter) in untreated women with stage III or IV ovarian cancer thathad been surgically debulked subop-timally.79,85 There were major re-sponses in 64 and 77 percent ofthe women in the cyclophosphamide and paclitaxel groups, respectively (P ϭ0.025), and paclitaxel was asso-ciated with a small improvement in surgically defined complete respons-es (26 percent, as compared with 19percent; P ϭ0.08). The paclitaxelregimen reduced the risk of recurrence by 32 percent,and the median duration of progression-free survival was 13.8 and 17.9 months for women receiving cyclo-phosphamide and paclitaxel, respectively; however, the duration of follow-up was too short to permit the as-sessment of overall survival. Although the incidence of severe neutropenia was higher in the paclitaxel arm,there was no increase in sepsis. This study suggests that the combination of paclitaxel and cisplatin will be-come the new standard therapy for advanced ovarian cancer.Two important issues — whether the schedule of ad-ministration of paclitaxel (short vs. long infusion) is im-portant from either a toxicologic or a therapeutic standpoint and whether there is a dose–response rela-tion in the usual dosing range — are being studied inwomen with ovarian cancer. As previously discussed,the effects of two paclitaxel doses (135 and 175 mg per square meter) and two schedules (24-hour and 3-hour infusions) with premedication for hypersensitivity reac-tions were similar.45 Progression-free survival was sig-nificantly longer in the high-dose group than in the low-dose group (19 vs. 14 weeks, P ϭ0.02), but survivalwas similar in both dose and schedule groups. Al-though regulatory approval was originally granted for the use of paclitaxel at a dose of 135 mg per square meter on a 24-hour schedule in women with drug-refractory and recurrent ovarian cancer, these resultswere the impetus for the subsequent approval of doses of 175 mg per square meter of paclitaxel administered*NA denotes not available, and G-CSF granulocyte colony-stimulating factor.†A complete response is defined as the complete disappearance of tumor present before treatment. A partial response isdefined as a reduction by at least 50 percent in the sum of the bidimensional measurements of all known sites of disease.‡Response rate is defined as the percentage of patients who could be evaluated who had either complete or partial re-sponses. Platinum resistance is defined as tumor progression while receiving or within six months after completing therapywith a platinum-containing chemotherapy regimen.§Value represents pathologically verified complete responses.¶These totals do not include previous platinum-response data from the Einstein Medical Center and National Cancer In-stitute phase 2 studies since this information was not provided in the original reports.81,82ʈThese specific data were not provided in the original reports.81,82Eighty-nine percent of all patients participating in theNational Cancer Institute phase 2 study of paclitaxel and G-CSF had platinum-resistant ovarian cancer.82**This is an open-enrollment program permitting compassionate use of paclitaxel in patients with resistance to platinum.Table 3. Early Evaluations of Paclitaxel in Women with Advanced and RefractoryOvarian Carcinoma.*S TUDY N O . OFP ATIENTS M AJOR R ESPONSES †R ESPONSE R ATE ‡COM-PLETE PARTIAL OVERALL PLATINUM-SENSITIVE PLATINUM-RESISTANTnumber %no. with response/total no. (%)Phase 2 single-agent studies McGuire et al.50401§11306/15 (40)6/25 (24)Thigpen et al.544388377/16 (44)9/27 (33)Einzig et al.81301§5203/NA¶3/NA¶ʈPhase 1 study of paclitaxel ϩ G-CSFSarosy et al.381414360/35/11 (45)Phase 2 study of paclitaxel ϩ G-CSFKohn et al.824461548NA¶ʈNA¶ʈAll studies 17117433513/34 (38)¶20/63 (32)¶Treatment referral center program **Trimble et al.556522311822—141/652 (22)。
